Sernova Commercial Sernova Commercial
About Sernova, Corp. À propos de Sernova, Corp.
Sernova is committed to the development and clinical advancement of its products for metabolic, hematological and other chronic diseases using therapeutic cells transplanted into a patented implanted medical device, which forms an organ-like environment promoting long-term function and survival of the therapeutic cells. Sernova s'engage dans le développement et l'avancement clinique de ses produits contre les maladies métaboliques, hématologiques et autres maladies chroniques utilisant des cellules thérapeutiques transplantées dans un dispositif médical implanté breveté, qui forme un environnement semblable à un organe favorisant la fonction et la survie à long terme des cellules thérapeutiques.
The company’s management believes in building strong and long-lasting collaborations and partnerships that would lead to the rapid advancement of Sernova’s portfolio of products into the market, improving global health and bringing value to patients and society, in concert with our clinical development programs. La direction de la société croit à la mise en place de collaborations et de partenariats solides et durables qui permettraient au portefeuille de produits de Sernova de progresser rapidement sur le marché, d’améliorer la santé mondiale et d’apporter une valeur ajoutée aux patients et à la société, de concert avec nos programmes de développement clinique.
Indications: Les indications:
Diabetes Diabète
Haemophilia A Hémophilie A
Thyroid disease Maladie thyroïdienne
Clinical studies: Etudes cliniques:
Diabetes US phase I/II clinical study cleared by the FDA Etude clinique de phase I / II sur le diabète, approuvée par la FDA
First-in-human study in Diabetic subjects with hypoglycemia unawareness Première étude chez l'homme chez des sujets diabétiques peu conscients de l'hypoglycémie
Cell Pouch System™
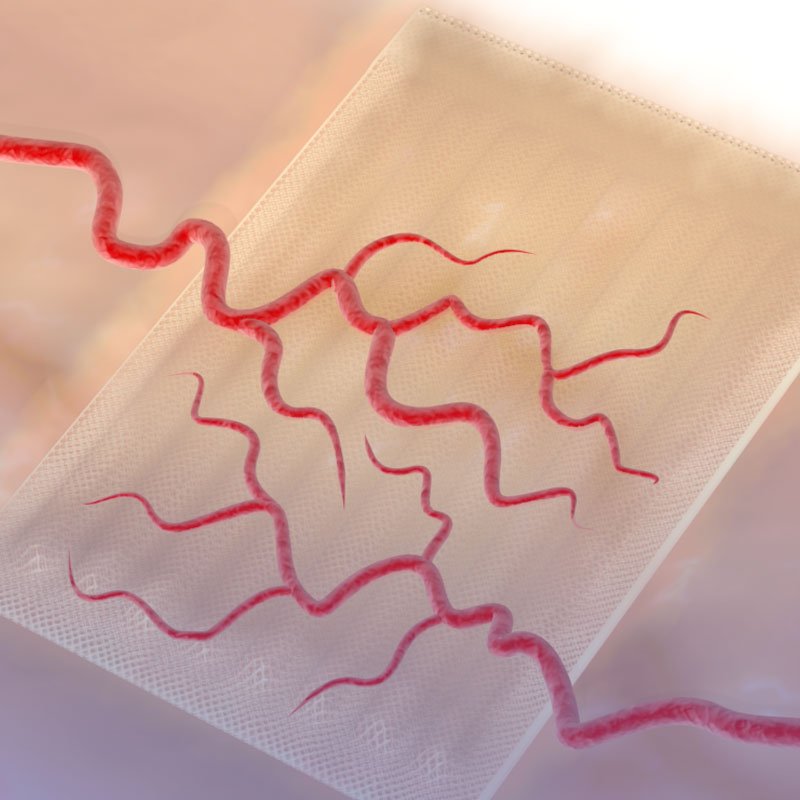
Sernova’s Cell Pouch System™ is a novel implantable and scalable medical device which forms a highly vascularized organ-like environment in the body for the housing, function and long-term survival of therapeutic cells. These therapeutic cells release necessary proteins or hormones missing from the body to treat chronic diseases as an alternative to daily administration of drugs. Le Cell Pouch System™ est un nouveau dispositif médical préalablement implanté et formant un environnement naturel hautement vascularisé afin d’y loger des cellules thérapeutiques, favorisant leur bon fonctionnement et leur survie dans le corps. Ces cellules thérapeutiques libèrent les protéines ou les hormones nécessaires pour traiter les maladies chroniques comme alternative a l’administration quotidienne de médicaments.

Immune Protection Protection immunitaire
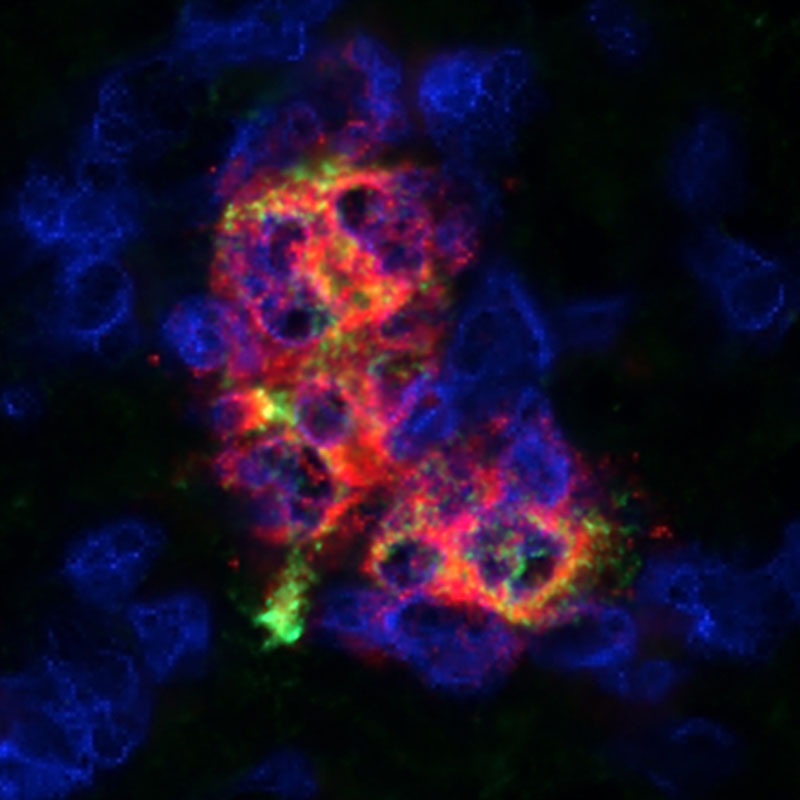
We have shown that cells can be protected using medications that prevent immune system attack within the Cell Pouch™. Nous avons montré que les cellules peuvent être protégées en utilisant des médicaments qui empêchent les attaques du système immunitaire dans la Cell Pouch™.
Microencapsulation technologies house cells within the Cell Pouch chambers and protect the cells from immune system attack. Les technologies de microencapsulation hébergent les cellules dans les chambres à cellules et les protègent des attaques du système immunitaire.
Technologies are in development to make transplanted cells unrecognizable to the immune system. Des technologies sont en cours de développement pour rendre les cellules greffées méconnaissables du système immunitaire.
Sernova’s Cell Pouch™, combined with immune protected therapeutic cells, offers protection from immune system attack creating an effective, safe, long-term and convenient therapeutic option for patients with chronic diseases who seek to improve their quality of life. Le Cell Pouch™ de Sernova, associé à des cellules immunitaires protégées, offre une protection contre le système immunitaire avec une option thérapeutique efficace, sûre, à long terme et pratique pour les patients atteints de maladies chroniques qui cherchent à améliorer leur qualité de vie.

Featured News Nouvelles en vedette
Sernova Business Update
Marek Sutherland of CTV News London - Cure for type one diabetes getting closer, London company says
Noah Stansfield of CGT Live - Patients With T1D Achieve Insulin Independence Following Implantation of Cell Pouch System and Islet Transplant
Sean Whooley and Danielle Kirsh of Fast Five- Teleflex has a Class I recall, Boston Scientific appoints two new board directors (Sernova discussed in the podcast recording from 1:00-2:58 time marks)
Lei Lei Wu of EndPoints News - Sernova says five diabetes patients have now been taken off insulin after 'cell pouch' therapy
Shane Whooley of MassDevice - Sernova reports positive interim data for Cell Pouch System
News Releases Communiqués de presse
Sernova Announces Key New Executive Appointments
Sernova to Present at the 2024 Cell & Gene Meeting on the Mesa
Sernova Appoints David Paterson Ph.D. to its Board of Directors
Sernova Announces New Positive Data from Phase I/II Trial Regarding Islet Survival and Function
Updates Mises à jour
Sernova KOL Event on Thyroid Disease with Dr. Sam Wiseman
Watch now!
Visionner maintenant!
Events Événements
Sernova Corporate Presentation at H.C Wainwright Conference Sernova Corporate Presentation at H.C Wainwright Conference
Sernova AGM - Annual General Meeting Sernova AGM - Annual General Meeting
2024 Bloom Burton & Co. Healthcare Investor Conference 2024 Bloom Burton & Co. Healthcare Investor Conference
Invest with Sernova Investir dans Sernova
If you are a shareholder, investor, broker, analyst, journalist, investment advisor, or looking to develop business opportunities, please feel free to contact us by email or telephone. Si vous êtes actionnaire, investisseur, courtier, analyste, journaliste, conseiller en placement ou souhaitez développer des opportunités d’affaires, n'hésitez pas à nous contacter par email ou par téléphone.
Sernova is a regenerative medicine company developing therapeutic technologies with multibillion-dollar market potential for each of its clinical indications. Sernova est une société de médecine régénérative développant des technologies thérapeutiques offrant un potentiel de marché de plusieurs milliards de dollars pour chacune de ses indications cliniques.
Sernova is a Collaborative Team Sernova, c’est aussi les collaborations
We believe in advancing our clinical programs and building strong and long-lasting collaborations and partnerships that will lead to the rapid advancement of Sernova’s portfolio of products into the market, improving global health and bringing value to patients and society. Nous croyons en la promotion de nos programmes cliniques et en la mise en place de collaborations et de partenariats solides et durables qui permettront au portefeuille de produits de Sernova de progresser rapidement sur le marché, d'améliorer la santé mondiale et d'apporter de la valeur aux patients et à la société.
Privacy PolicyPolitique de confidentialité
Updated July 6, 2018
Please read this Policy carefully along with our Legal Notice that describes our Terms of Use for the Website.
By accessing www.sernova.com (the “Website”) you hereby agree with the practices described in this Privacy Policy (the “Policy”)
This Policy applies to all information gathered through the Website and/or any related marketing technique or events.
Information Collection
The information collected is limited to the information that you decide to share with us through the News Dispatch Service, when participating at event or activities or in the general course of business by expressing an interest in obtaining information about Sernova Corp. and our products, such as name, email, phone number, and similar contact information. This information is stored through MailChimp (please refer to MailChimp Privacy Policy at https://mailchimp.com/legal/privacy/).
Information Sharing
Sernova Corp. is the sole owner of any information collected on the Website. We do not sell, share or rent this information to others.
Traffic and Automatic Information Collection
Sernova Corp. maintains log files of the traffic on www.sernova.com. This information is not linked to any personal information that you have provided us. Logs are used to manage traffic, identify content accessed, and IT requirements. Information logged and automatically collected includes without being limited to IP addresses and browser types. This information does not reveal your specific identity.
Cookies
Cookies can be used to provide you with a more personalized experience. The Website may use cookies to make that experience more companionable when you return to the Website. You have the option at all time to decline the use of cookies. If you choose to do so, you may not be able to fully use all features of the Website. You can also delete cookie files at all time from your computer. Those cookies may include first-party cookies (such as the Google Analytics cookies).
Updates
This Policy is a living document and may be amended or updated from time to time without further notice. We encourage you to review the Policy periodically.
Contact
If you have any questions or comments about our policy, you can email us at info@sernova.com or by phone at 1(877) 299-4603 or by mail at
Sernova Corp.
700 Collip Circle, Suite 114
London, ON Canada N6G 4X8
Mis à jour le 6 juillet 2018
Veuillez lire attentivement cette politique ainsi que notre avis juridique qui décrit nos conditions d'utilisation du site Web.
En accédant à www.sernova.com (le «site Web»), vous acceptez les pratiques décrites dans la présente politique de confidentialité (la «politique»).
Cette politique s'applique à toutes les informations collectées via le site Web et / ou toute technique ou événement marketing associé.
Collecte d'informations
Les informations collectées se limitent aux informations que vous décidez de partager avec nous par le biais du service d’expédition de nouvelles, lorsque vous participez à un événement ou à des activités ou que vous vous intéressez à obtenir des informations sur Sernova Corp. comme nom, email, numéro de téléphone et informations de contact similaires. Ces informations sont stockées via MailChimp (veuillez vous reporter aux règles de confidentialité de MailChimp sur https://mailchimp.com/legal/privacy/).
Partage d'information
Sernova Corp. est l'unique propriétaire de toute information collectée sur le site Web. Nous ne vendons pas, ne partageons pas ou ne louons pas ces informations à des tiers.
Collecte d'informations routières et automatiques
Sernova Corp. gère les fichiers journaux du trafic sur www.sernova.com. Ces informations ne sont liées à aucune information personnelle que vous nous avez fournie. Les journaux sont utilisés pour gérer le trafic, identifier le contenu accédé et les besoins informatiques. Les informations consignées et collectées automatiquement ne sont pas limitées aux adresses IP et aux types de navigateur. Cette information ne révèle pas votre identité spécifique.
Cookies
Les cookies peuvent être utilisés pour vous offrir une expérience plus personnalisée. Le site Web peut utiliser des cookies pour rendre cette expérience plus conviviale lorsque vous revenez sur le site Web. Vous avez la possibilité à tout moment de refuser l'utilisation de cookies. Si vous choisissez de le faire, vous ne pourrez peut-être pas utiliser toutes les fonctionnalités du site Web. Vous pouvez également supprimer des fichiers de cookies à tout moment depuis votre ordinateur. Ces cookies peuvent inclure des cookies de première partie (tels que les cookies de Google Analytics).
Mises à jour
Cette politique est un document évolutif et peut être modifié ou mis à jour de temps à autre sans préavis. Nous vous encourageons à consulter la politique périodiquement.
Contact
Si vous avez des questions ou des commentaires sur notre politique, vous pouvez nous envoyer un courriel à info@sernova.com ou par téléphone au 1 (877) 299-4603 ou par courrier à
Sernova Corp.
700 Collip Circle, Suite 114
London, ON Canada N6G 4X8
Press Release Communiqué de presse - October 11, 2024 11 October, 2024
Sernova Announces Key New Executive Appointments
Experienced leadership team positions company to advance Cell Pouch™ bio-hybrid organ programs.
LONDON, Ontario; BOSTON, Massachusetts - October 11, 2024 – Sernova Corp. (TSX: SVA) (OTCQB:
SEOVF) (FSE/XETRA: PSH), a leading regenerative medicine company currently executing a phase 1/2
clinical study with its’ Cell Pouch™ bio-hybrid organ in type 1 diabetes is pleased to announce key new
appointments to its executive leadership team. This strategic move reflects Sernova's commitment to
enhancing communication with stakeholders and corporate governance, as the company drives its’
innovative research and ongoing clinical development programs.
James Parsons has been named Chief Financial Officer (CFO), bringing over 20 years of financial
management experience to the team. With a comprehensive track record in biotech corporate finance,
governance and strategic planning, Mr. Parsons will lead Sernova's finance team and ensure the company’s
financial strength to support expanding objectives.
Joining as Chief Communications Officer (CCO) is Marylyn Rigby, an accomplished professional with a rich
history in branding, corporate communications and biotech and health tech marketing. Ms. Rigby will direct
Sernova’s comprehensive communication strategies, enhancing marketing initiatives, expanding media
relationships, and elevating investor and stakeholder engagement, pivotal for bolstering Sernova's brand
identity and outreach.
David Burke assumes the role of Vice President of Investor Relations, tasked with fortifying connections with
the investment community. Bringing extensive experience in investor communications and financial markets,
Mr. Burke will ensure transparent, timely, and effective dialogue with current and prospective investors,
building trust and fostering long-term support for Sernova’s visionary projects.
Jonathan Rigby, CEO of Sernova Corp., expressed his enthusiasm for the appointments, stating, "We are
thrilled to welcome James, Marylyn, and David to our leadership team. Each brings a unique set of skills
and deep industry knowledge that will be vital as we continue to advance our pipeline and expand our
corporate presence. Their expertise aligns perfectly with our mission to Give Patients Their Lives Back
through our groundbreaking therapies."
These key appointments exemplify a step change in Sernova’s unwavering commitment to excellence and
innovation as it continues to drive forward novel treatments that aim to transform how chronic conditions are
treated in order to improve the lives of patients, their families, and their caregivers.
ABOUT SERNOVA CORP
Sernova Corp. is a clinical-stage company developing regenerative medicine therapeutics combining its Cell
Pouch™ with human donor cells or stem cells to create a bio-hybrid organ. A bio-hybrid organ refers to a
medical device designed to be implanted into the human body, where it integrates with existing living tissue
to replicate or enhance the function of a natural organ, essentially aiming to restore normal organ function
by combining living cells with non-living materials to mimic the properties of the original organ and
seamlessly interact with surrounding tissues. This innovative approach aims to deliver a potentially
revolutionary treatment for patients with chronic diseases, initially focusing on type 1 diabetes and thyroid
disorders.
FOR FURTHER INFORMATION, PLEASE CONTACT:
David Burke
VP, Investor Relations
(917) 618-2651
Email: David.Burke@sernova.com
Website: Sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or adequacy
of this news release.
FORWARD-LOOKING INFORMATION
This press release contains forward-looking statements within the meaning of applicable Canadian securities
laws. Forward-looking statements in this press release include our expectations of the positive impact our
management changes will have on our operations.
With respect to the forward-looking statements contained in this press release, Sernova has made numerous
assumptions regarding, among other things: the company’s ability to secure additional financing on
reasonable terms, or at all; and the ability to conduct all required preclinical and clinical studies for the
company’s Cell Pouch, including the timing and results of those trials. A more complete discussion of the
risks and uncertainties facing Sernova appears in Sernova’s Annual Information Form for the year ended
October 31, 2023 filed with Canadian securities authorities and available at www.sedarplus.ca, as updated
by Sernova’s continuous disclosure filings, which are available at www.sedarplus.ca. All forward-looking
statements herein are qualified in their entirety by this cautionary statement, and Sernova disclaims any
obligation to revise or update any such forward-looking statements or to publicly announce the result of any
revisions to any of the forward-looking statements contained herein to reflect future results, events or
developments, except as required by law.
Press Release Communiqué de presse - October 03, 2024 3 October, 2024
Sernova to Present at the 2024 Cell & Gene Meeting on the Mesa
LONDON, Ontario; BOSTON, Massachusetts – October 3, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative
medicine cell therapies for treatment of chronic diseases, announced that its CEO, Jonathan Rigby, will be
presenting at the 2024 Cell & Gene Meeting on the Mesa being held October 7-9, 2024, at the Arizona Biltmore
Hotel in Phoenix, Arizona. The Cell & Gene Meeting on the Mesa is the sector’s foremost annual conference
bringing together senior executives and top decision-makers in the industry to advance cutting-edge research
into cell therapy cures.
Mr. Rigby’s presentation is scheduled to begin at 10:00 A.M. MT (12:00 P.M. ET) on Monday, October 7, 2024.
The presentation will include updates on Sernova’s type 1 diabetes phase 1/2 clinical trial at the University of
Chicago, and its pre-clinical work with Evotec to advance a potential iPSC-based (induced pluripotent stem cell)
islet replacement therapy with Sernova’s Cell Pouch™ Transplant System.
ABOUT SERNOVA AND ITS CELL POUCH™ TRANSLPLANT SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes with
its lead technology, the Cell Pouch Transplant System, a novel implantable and scalable medical device with
immune protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-term
survival and function of therapeutic cells that release essential factors that are absent or deficient in patients with
certain chronic diseases. Sernova’s Cell Pouch Transplant System has demonstrated its potential to be a
‘functional cure’ for people with T1D in an ongoing Phase I/II clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch Transplant System also includes: a cell therapy for hypothyroid
disease resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
David Burke
VP, Investor Relations
Sernova Corp.
Tel: +1 917-618-2651
Email: david.burke@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or adequacy of
this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but not
always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects", "potential
for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should" occur are
used to identify forward-looking statements. These statements reflect management’s beliefs with respect to
future events and are based on information currently available to management on the date such statements were
made. Many factors could cause Sernova’s actual results, performances or achievements to not be as
anticipated, estimated or intended or to differ materially from those expressed or implied by the forward-looking
statements contained in this news release. Such factors could include, but are not limited to, the company’s
ability to secure additional financing and licensing arrangements on reasonable terms, or at all; ability to conduct
all required preclinical and clinical studies for the company’s Cell Pouch System and or related technologies,
including the timing and results of those trials; ability to obtain all necessary regulatory approvals, or on a timely
basis; ability to in-license additional complementary technologies; ability to execute its business strategy and
successfully compete in the market; and the inherent risks associated with the development of biotechnology
combination products generally. Many of the factors are beyond our control, including those caused by, related
to, or impacted by the novel coronavirus pandemic. Investors should consult the company’s quarterly and annual
filings available on www.sedarplus.ca for additional information on risks and uncertainties relating to the
forward-looking statements. Sernova expressly disclaims any intention or obligation to update or revise any
forward-looking statements, whether as a result of new information, future events or otherwise.
Press Release Communiqué de presse - September 19, 2024 19 September, 2024
Sernova Appoints David Paterson Ph.D. to its Board of Directors
Sernova Appoints David Paterson Ph.D. to its Board of Directors
LONDON, Ontario; BOSTON, Massachusetts – September 19, 2024 – Sernova Corp. (TSX:SVA)
(OTCQB:SEOVF) (FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of
regenerative medicine cell therapies for treatment of chronic diseases, announces today that David Paterson
Ph.D. will join Sernova’s Board of Directors effective immediately.
“I am pleased to have been able to attract David’s Paterson’s significant talent to Sernova’s Board of Directors,”
said Jonathan Rigby, President and Chief Executive Officer of Sernova. “Mr. Paterson brings more than 30 years
of biotech experience with substantial contributions as both a senior executive, Board member and founder of
numerous companies with a focus on corporate and business development, intellectual property generation
and prosecution and alliance management.”
Mr. Paterson is currently employed at Colorado State University as Assistant Vice President for Research
Translation and Commercialization where he manages strategic industry relationships. In addition, he brings
broad biopharma industry knowledge having worked for Impax Laboratories (IPXL: NASDAQ) in both Europe
and North America where he provided leadership and Board support to the business development team for ten
years until its acquisition by Amneal Pharmaceuticals (AMRX: NASDAQ). Prior to Impax, Mr. Paterson held
senior roles with Sepracor (Sunovion), GlaxoSmithKline and Skyepharma. Mr. Paterson is also a co-founder of
Neurogastrx, Inc helping to bring in early-stage capital. Mr. Paterson has a Batchelor of Science from the
University of Glasgow, a Ph.D. from the University of Illinois and was a Post-Doctoral Fellow in Molecular
Genetics and Cellular Biology at the University of Chicago.
“I am very excited to be joining the Board of Sernova and offer up my scope of expertise to help the company
grow and prosper within the type 1 diabetes space and to help open doors to new collaborations and further
establish the Cell Pouch Transplantation System as a top tier technology within the Regenerative Medicine Cell
Therapy sector,” said David Paterson.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
On implantation, The Cell Pouch forms a natural vascularized tissue environment in the body for long-term
survival and function of therapeutic cells that release essential factors that are absent or deficient in the bodies
of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a
‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or adequacy of
this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - September 12, 2024 12 September, 2024
Sernova Announces New Positive Data from Phase I/II Trial Regarding Islet Survival and Function
Sernova Announces New Positive Data from Phase I/II Trial Regarding Islet Survival and Function
- Abundant, richly vascularized and functioning islets observed throughout all chambers of Sernova’s Cell Pouch
more than 5 years after islet transplantation
- Histological data confirmation of healthy beta, alpha and delta cells secreting insulin, glucagon, and
somatostatin in all Cell Pouch Chambers
- Sernova’s Cell Pouch safely contains its therapeutic cells and provides full retrievability using conventional
instruments and methods
- Pathology confirmed no evidence of detrimental fibrotic tissue associated with Cell Pouch more than 5 years
after implantation
LONDON, Ontario; BOSTON, Massachusetts – September 12, 2024 – Sernova Corp. (TSX:SVA)
(OTCQB:SEOVF) (FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of
regenerative medicine cell therapies for treatment of chronic diseases today announced the presentation of new
positive interim data from the ongoing Phase I/II clinical trial evaluating the safety, tolerability, and efficacy of
the Sernova Cell Pouch Transplant System containing donor islets in people living with type 1 diabetes (T1D).
The data was presented by Piotr Witkowski, M.D., Ph.D., Professor of Surgery and Director of the Pancreatic
and Islet Transplant Program at University of Chicago Medicine, who is the Lead Investigator for the study,
during an oral session at the 2024 European Association for the Study of Diabetes (EASD) Annual Meeting in
Madrid, Spain.
All 6 of the patients enrolled in Cohort A of Sernova’s Phase I/II clinical trial with Cell Pouch and donor islets
achieved sustained insulin independence after combined islet transplantation into Cell Pouch and intraportally.
The first patient to be treated in the trial experienced sustained insulin independence for more than 4 years
accompanied by blood sugar levels in the non-diabetic range (HbA1c ≤6.5%). More than 5 years after the first
islet transplant, the patient’s Cell Pouches containing the transplanted islets were removed because immune
suppression had to be stopped when that patient developed other, nondiabetic health issues not related to Cell
Pouch or transplanted islets.
The new histological data from those explanted Cell Pouches confirmed abundant, well-vascularized,
functioning islets consisting of cells producing insulin, glucagon and somatostatin, throughout all chambers,
more than 5 years after being transplanted to Cell Pouch. Additionally, after being in the body for more than 5
years, a pathology examination confirmed there was no evidence of detrimental fibrotic tissue, material
degradation or changes in the architecture of the Sernova Cell Pouch.
“I am excited to see this evidence of well-vascularized and healthy islets 5 years after transplant to Sernova’s
Cell Pouch; these interim findings are very promising,” commented Piotr Witkowski M.D., Ph.D. “This is a major
step forward in the development of a contained and retrievable cell therapy for the treatment of T1D. This is
the first evidence that I am aware of that demonstrates this level of healthy islet survival and function in an
implantable and retrievable system for such a long duration.”
“We believe this first-in-world data is significant for Sernova and, more specifically, provides tangible hope for
T1D patients that we are a significant step further in our mission of providing a functional cure for this terrible
disease; as a Type 1 diabetic myself I could not be more determined to drive our program forward and
ultimately onto the market,” said Jonathan Rigby, President and CEO of Sernova. “We look forward to
completing Cohort B in the near term and, based on positive data generated thus far, initiating Cohort C of our
ongoing trial later this year with an optimized immune suppression regimen. Lastly, we continue to work with
our partner Evotec on the development of induced pluripotent stem cell (iPSC)-derived islet-like clusters, which
will provide a scalable cell source so that one day we can give patients with T1D their lives back”
ABOUT THE PHASE I/II TRIAL
The Phase I/II trial (NCT03513939) is a U.S. prospective, single-arm, multi-cohort study evaluating the safety,
tolerability, and efficacy of Sernova’s Cell Pouch in combination with transplanted human-donor islets in people
living with type 1 diabetes (T1D). The trial includes participants aged 18-65 with T1D who experience
hypoglycemic unawareness and severe hypoglycemic episodes, and who are eligible for donor islet
transplantation. The trial is currently divided into two cohorts. Cohort A involved six patients who received the
first-generation 8-channel Cell Pouch. Cohort B is evaluating seven patients transplanted with an optimized 10-
channel Cell Pouch, which has a 50% greater islet capacity than the Cell Pouch used in Cohort A. As part of the
process, patients are implanted with the Cell Pouch subcutaneously. Approximately six weeks later - allowing
time to establish a stable immunosuppression therapy for the patient - islets are transplanted into the
prevascularized tissue chambers formed by the Cell Pouch. Safety and efficacy are assessed throughout the 12
months following the last islet transplant. Patients still dependent on insulin 6 months after the second islet
transplant may qualify for a third transplant via the portal vein. Those who retain implants will be followed for
at least three years. The secondary objectives of the trial include the following efficacy endpoints: continuous
glucose monitoring, production of C-peptide, insulin use, HbA1c levels, and the frequency of severe
hypoglycemic episodes.
ABOUT SERNOVA AND ITS CELL POUCH™ TRANSPLANT SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch Transplant System, a novel implantable and scalable medical device
with immune protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in
patients with certain chronic diseases. Sernova’s Cell Pouch Transplant System has demonstrated its potential
to be a ‘functional cure’ for people with T1D in an ongoing Phase I/II clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch Transplant System also includes: a cell therapy for hypothyroid
disease resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or
adequacy of this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - September 05, 2024 5 September, 2024
Sernova to Participate in Upcoming H.C. Wainwright Investor Conference
Sernova to Participate in Upcoming H.C. Wainwright Investor Conference
LONDON, Ontario – September 5, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF) (FSE/XETRA:PSH), a
clinical- stage company and leader in cell therapeutics, today announced it will be participating in the
upcoming H.C. Wainwright 26th Annual Global Investment Conference being held September 9 to 11, 2024
in New York City at the Lotte New York Palace. Company management will also be participating in one-on-
one investor meetings at the conference.
Sernova’s presentation will be webcast, details as follows:
Sernova Corporate Presentation Date & Time: 11:00AM ET, September 11, 2024 presented by Jonathan
Rigby, President & Chief Executive Officer, Sernova Corp Location:
https://journey.ct.events/view/c4b9e5c3-766e-4c11-a345-bedcacb42ce8
Please contact your representative at H.C. Wainwright to schedule a one-on-one meeting with the
management team during the conference.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes and thyroid disease. Sernova is currently focused on
developing a ‘functional cure’ for insulindependent diabetes with its lead technology, the Cell Pouch System,
a novel implantable and scalable medical device with immune protected therapeutic cells. On implantation,
The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long- term survival and
function of therapeutic cells that release essential factors that are absent or deficient in patients with certain
chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a ‘functional cure’ for
people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago. Sernova is collaborating
with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet
replacement therapy. This partnership is expected to provide Sernova a potentially unlimited supply of
insulin-producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2).
Sernova’s Cell Pouch System development pipeline also includes a cell therapy for hypothyroid disease
resulting from thyroid gland removal, and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the
accuracy or adequacy of this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible,
but not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates",
"projects", "potential for" and similar expressions, or that events or conditions "will", "would", "may",
"could" or "should" occur are used to identify forward-looking statements. These statements reflect
management’s beliefs with respect to future events and are based on information currently available to
management on the date such statements were made. Many factors could cause Sernova’s actual results,
performances or achievements to not be as anticipated, estimated or intended or to differ materially from
those expressed or implied by the forwardlooking statements contained in this news release. Such factors
could include, but are not limited to, the company’s ability to secure additional financing and/or licensing
arrangements on reasonable terms, or at all; conduct all required preclinical and clinical studies for the
company’s Cell Pouch System and or related technologies, including the timing and results of those trials;
obtain all necessary regulatory approvals, or on a timely basis; in-license additional complementary
technologies; execute its business strategy and successfully compete in the market; and the inherent risks
associated with the development of biotechnology combination products generally. Many of the factors are
beyond our control, including those caused by, related to, or impacted by the novel coronavirus pandemic.
Investors should consult the company’s quarterly and annual filings available on www.sedarplus.ca for
additional information on risks and uncertainties relating to the forward-looking statements. Sernova
expressly disclaims any intention or obligation to update or revise any forward-looking statements, whether
as a result of new information, future events or otherwise.
Press Release Communiqué de presse - September 04, 2024 4 September, 2024
Sernova Closes $5.2 Million Over-subscribed Non-brokered, Private Placement
SERNOVA CLOSES $5.2 MILLION OVER-SUBSCRIBED NON-BROKERED, PRIVATE PLACEMENT
LONDON, ONTARIO – September 4, 2024 - Sernova Corp. (TSX: SVA) (OTCQB: SEOVF) (FSE: PSH) is
pleased to announce that it has closed its non-brokered, private placement in the amount over $5.2 million,
which includes over-subscriptions of more than $1.2 million.
All securities issued pursuant to the private placement are subject to a hold period of four months under
applicable provincial securities laws in Canada. The private placement was announced on August 20, 2024,
and closed on September 3, 2024.
On Thursday September 12 at the 2024 EASD Annual Meeting in Madrid, Spain, Sernova’s principal clinical
study investigator will provide an update on recent data generated from its phase I/II clinical study of Cell
Pouch™ containing human donor islets for the treatment of type 1 diabetes.
This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities
in the United States. The securities have not been and will not be registered under the United States
Securities Act of 1933, as amended (the “U.S. Securities Act”) or any state securities laws and may not be
offered or sold within the United States unless registered under the U.S. Securities Act and applicable state
securities laws or an exemption from such registration is available.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes and thyroid disease. Sernova is currently focused on
developing a ‘functional cure’ for insulindependent diabetes with its lead technology, the Cell Pouch System,
a novel implantable and scalable medical device with immune protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in
patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a
‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova is collaborating with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem
cells) based islet replacement therapy. This partnership is expected to provide Sernova a potentially
unlimited supply of insulin-producing cells to treat millions of patients with insulin-dependent diabetes (type
1 and type 2). Sernova’s Cell Pouch System development pipeline also includes a cell therapy for
hypothyroid disease resulting from thyroid gland removal, and an ex vivo lentiviral Factor VIII gene therapy
for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or adequacy
of this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible,
but not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates",
"projects", "potential for" and similar expressions, or that events or conditions "will", "would", "may",
"could" or "should" occur are used to identify forward-looking statements. These statements reflect
management’s beliefs with respect to future events and are based on information currently available to
management on the date such statements were made. Many factors could cause Sernova’s actual results,
performances or achievements to not be as anticipated, estimated or intended or to differ materially from
those expressed or implied by the forwardlooking statements contained in this news release. Such factors
could include, but are not limited to, the company’s ability to secure additional financing and/or licensing
arrangements on reasonable terms, or at all; conduct all required preclinical and clinical studies for the
company’s Cell Pouch System and or related technologies, including the timing and results of those trials;
obtain all necessary regulatory approvals, or on a timely basis; in-license additional complementary
technologies; execute its business strategy and successfully compete in the market; and the inherent risks
associated with the development of biotechnology combination products generally. Many of the factors are
beyond our control, including those caused by, related to, or impacted by the novel coronavirus pandemic.
Investors should consult the company’s quarterly and annual filings available on www.sedarplus.ca for
additional information on risks and uncertainties relating to the forward-looking statements. Sernova
expressly disclaims any intention or obligation to update or revise any forward-looking statements, whether
as a result of new information, future events or otherwise.
Press Release Communiqué de presse - August 29, 2024 29 August, 2024
Sernova Announces Oversubscribed Non-brokered Private Placement
SERNOVA ANNOUNCES OVERSUBSCRIBED NON-BROKERED PRIVATE PLACEMENT
THIS NEWS RELEASE IS NOT INTENDED FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR
DISSEMINATION IN THE UNITED STATES.
LONDON, ONTARIO - August 29, 2024 - Sernova Corp. (TSX: SVA) (OTCQB: SEOVF) (FSE: PSH) is pleased to
announce that its non-brokered, private placement offering of $4M is oversubscribed with $4.7M of binding
agreements received. The closing will occur on Tuesday September 3, 2024, subject to TSX approval.
This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities in
the United States. The securities have not been and will not be registered under the United States Securities Act
of 1933, as amended (the “U.S. Securities Act”) or any state securities laws and may not be offered or sold
within the United States unless registered under the U.S. Securities Act and applicable state securities laws or
an exemption from such registration is available.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes and thyroid disease. Sernova is currently focused on
developing a ‘functional cure’ for insulindependent diabetes with its lead technology, the Cell Pouch System, a
novel implantable and scalable medical device with immune protected therapeutic cells. On implantation, The
Cell Pouch forms a natural, vascularized tissue environment in the body allowing long- term survival and
function of therapeutic cells that release essential factors that are absent or deficient in patients with certain
chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a ‘functional cure’ for
people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago. Sernova partnered with
Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet replacement
therapy. This partnership provides Sernova a potentially unlimited supply of insulin-producing cells to treat
millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s development pipeline that
uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease resulting from thyroid gland
removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
The TSX has not reviewed this news release and does not accept responsibility for the accuracy or
adequacy of this news release.
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forwardlooking statements contained in this news release. Such factors could include, but are not limited to, the
company’s ability to complete the Offering, in part or at all; secure additional financing and/or licensing
arrangements on reasonable terms, or at all; conduct all required preclinical and clinical studies for the
company’s Cell Pouch System and or related technologies, including the timing and results of those trials;
obtain all necessary regulatory approvals, or on a timely basis; in-license additional complementary
technologies; execute its business strategy and successfully compete in the market; and the inherent risks
associated with the development of biotechnology combination products generally. Many of the factors are
beyond our control, including those caused by, related to, or impacted by the novel coronavirus pandemic.
Investors should consult the company’s quarterly and annual filings available on www.sedarplus.ca for
additional information on risks and uncertainties relating to the forward-looking statements. Sernova expressly
disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of
new information, future events or otherwise.
Press Release Communiqué de presse - August 20, 2024 20 August, 2024
Sernova Announces Up to $4 Million Private Placement Priced at a Premium
Sernova Announces Up to $4 Million Private Placement Priced at a Premium
THIS NEWS RELEASE IS NOT INTENDED FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR
DISSEMINATION IN THE UNITED STATES.
LONDON, ONTARIO - August 20, 2024 - Sernova Corp. (TSX: SVA) (OTCQB: SEOVF) (FSE: PSH) is pleased to
announce that the Company has secured over $2 million in lead orders in connection with a non-brokered
private placement offering (the “Offering”) for up to $4 million units priced at a premium to the market.
Each unit is priced at $0.25, and consists of one common share and one warrant. Each warrant is priced at
$0.30 per share and is exercisable for a period of 18 months, subject to Sernova’s option to shorten the exercise
period if the 20-day volume-weighted average price of the Company’s shares exceeds $0.50.
The net proceeds from the private placement will be used to continue enrolment in the Company's US-based
Phase I/II Type 1 diabetes clinical trial, to advance an IND filing for its post-operative hypothyroidism program
and for general corporate purposes.
Newly appointed Sernova CEO Jonathan Rigby stated, “We are pleased to announce a financing round led by
legacy investors with insider participation. Our goal is to secure sufficient capital to maintain ongoing clinical
initiatives. The company recently announced that data from its ongoing clinical trial of its Cell Pouch™
technology containing donor Islet cells to treat Type 1 diabetes will be presented at the European Association
for the Study of Diabetes (EASD), 9-13 September in Madrid, Spain.”
All securities issued in connection with the private placement will be subject to a statutory hold period of four
months. Completion of the private placement is subject to customary closing conditions, including acceptance
of the TSX. The private placement is expected to close prior to August 28, 2024.
The Company expects insider participation in the Offering, which may be considered a related party transaction
within the meaning of Multilateral Instrument 61-101 (“MI 61-101”). Sernova intends to rely on the exemptions
from the valuation and minority shareholder approval requirements of MI 61-101 contained in Sections 5.5(a)
and 5.7(1)(a) of MI 61-101 in respect of any insider participation.
This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities in
the United States. The securities have not been and will not be registered under the United States Securities Act
of 1933, as amended (the “U.S. Securities Act”) or any state securities laws and may not be offered or sold
within the United States unless registered under the U.S. Securities Act and applicable state securities laws or
an exemption from such registration is available.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes and thyroid disease. Sernova is currently focused on
developing a ‘functional cure’ for insulin-dependent diabetes with its lead technology, the Cell Pouch System, a
novel implantable and scalable medical device with immune protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in
patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a
‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to complete the Offering, in part or at all; secure additional financing and/or licensing
arrangements on reasonable terms, or at all; conduct all required preclinical and clinical studies for the
company’s Cell Pouch System and or related technologies, including the timing and results of those trials;
obtain all necessary regulatory approvals, or on a timely basis; in-license additional complementary
technologies; execute its business strategy and successfully compete in the market; and the inherent risks
associated with the development of biotechnology combination products generally. Many of the factors are
beyond our control, including those caused by, related to, or impacted by the novel coronavirus pandemic.
Investors should consult the company’s quarterly and annual filings available on www.sedarplus.ca for
additional information on risks and uncertainties relating to the forward-looking statements. Sernova expressly
disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of
new information, future events or otherwise.
Press Release Communiqué de presse - August 15, 2024 15 August, 2024
Lead Investigator for Sernova's Clinical Trial with Cell Pouch for Type 1 Diabetes to Deliver Oral Presentation at the 2024 EASD Annual Meeting
Lead Investigator for Sernova’s Clinical Trial with Cell Pouch for Type 1 Diabetes to Deliver Oral Presentation at
the 2024 EASD Annual Meeting
- Dr. Piotr Witkowski MD PhD, Professor of Surgery and Director of the Pancreatic and Islet Transplant Program,
and his islet transplant team at University of Chicago Medicine authored an abstract that will be presented,
including new data from the ongoing Phase I/II clinical trial of the Cell Pouch System™ in patients with type 1
diabetes (T1D).
LONDON, Ontario; BOSTON, Massachusetts – August 15, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative
medicine cell therapies for treatment of chronic diseases such as Type 1 Diabetes, today announced a short
oral presentation at the upcoming European Association for the Study of Diabetes (EASD) taking place
September 9-13, 2024 in Madrid, Spain.
Additional details, including accepted abstracts, are available on the EASD website at www.easd.org In
alignment with the embargo policy, Sernova plans to share details from Dr. Witkowski’s talk, following the
presentation.
Presentation details:
European Association for the Study of Diabetes - 2024 Annual Meeting – Madrid, Spain Oral Event F: Improving
Islet Transplantation - Thursday September 12, 2024 2:00pm to 3:00pm Central European Time
Abstract # 447: Islet allotransplantation into pre-vascularized Sernova Cell Pouch™: Interim Results: P.
Witkowski, N. Wojcik, S. Gondek, J. Tomecki, K. Milejczyk, B. Juengel, L. Wang, J. J. Fung, R. Barth, USA.
Sernova Corp continues to collaborate closely with leading academic, pharmaceutical and clinical institutions to
expand the scope and impact of its technology. The company anticipates further advancements as it progresses
through additional cohorts and trials, with the ultimate goal of offering a scalable solution for insulin-dependent
diabetes plus other chronic diseases.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in
patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a
‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - August 12, 2024 12 August, 2024
Sernova Corp. Announces Appointment of New Chief Executive Officer
SERNOVA CORP. ANNOUNCES APPOINTMENT OF NEW CHIEF EXECUTIVE OFFICER
LONDON, Ontario; BOSTON, Massachusetts – August 12, 2024. Sernova Corp. (“Sernova” or the
“Company”) (TSX:SVA) (OTCQB:SEOVF) (FSE/XETRA:PSH), a clinical-stage biotechnology company focused
on the development of regenerative medicine cell therapies for treatment of chronic diseases, led by an
ongoing phase 1/2a clinical trial in type 1 diabetes, is pleased to announce Mr. Jonathan Rigby as the
Company’s new Chief Executive Officer.
James Parsons, on behalf of the Board of Directors of Sernova, stated, “The Board believes that Sernova is
now positioned to achieve its strategic milestones under Jonathan’s leadership. Jonathan brings a track
record of success raising equity capital for biotech companies. Jonathan has led multiple biotech companies
through listings onto Nasdaq, and the achievement of key operational and clinical developments, leading to
strategic acquisitions that have generated significant value for shareholders.”
On his appointment, Mr. Rigby commented, “As a type 1 diabetic myself, I am honored to serve the company
and its shareholders as Sernova’s new CEO. I am passionate about Sernova’s mission and determined to lead
the Company to realize its full potential. I have financed and grown multiple companies through to exits and
my goal is clear; I will work tirelessly with the team to do the same for Sernova and its shareholders.”
Mr. Rigby has held several CEO roles and currently serves on the board of directors of cancer therapy
company, Oncolytics Biotech Inc. (Nasdaq: ONCY), and IM Therapeutics, working in the diabetes field. He
was formerly a board member of Xeris Pharmaceuticals (Nasdaq: XERS), which developed and
commercialized a product to treat type 1 diabetes severe hypoglycemia. Mr. Rigby holds a degree in
biological sciences from the University of Sheffield, U.K., and has a master of business administration degree
from the University of Portsmouth, U.K.
The Board thanks Ms. Cynthia Pussinen, the Company’s former CEO, for her dedication and service to the
Company and wishes her the very best in her future endeavors.
ABOUT SERNOVA CORP.
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies that
are implanted in patients inside its Cell PouchTM System for chronic diseases, including an ongoing phase
1/2a trial in insulin-dependent diabetes and plans to enter the clinic in thyroid disease, and blood disorders
that include hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-
dependent diabetes with its lead asset, the Cell Pouch System, a novel implantable and scalable medical
device with immune protected therapeutic cells. On implantation, the Cell Pouch forms a natural
vascularized tissue environment in the body for long-term survival and function of therapeutic cells that
release essential factors that are absent or deficient in the bodies of patients with certain chronic diseases.
Sernova’s Cell Pouch System has demonstrated its potential to be a ‘functional cure’ for people with T1D in
an ongoing Phase 1/2 clinical study at the University of Chicago. In May 2022, Sernova and Evotec entered
into a global strategic partnership to develop an implantable iPSC (induced pluripotent stem cells) based
Cell Pouch islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of
insulin-producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2).
Sernova continues to evaluate the potential for additional development programs that utilize its Cell Pouch
System: a cell therapy for hypothyroid disease resulting from thyroid gland removal and an ex vivo lentiviral
Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
christopher.barnes@sernova.com
519-902-7923
www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible,
but not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates",
"projects", "potential for" and similar expressions, or that events or conditions "will", "would", "may",
"could" or "should" occur are used to identify forward-looking statements. These statements reflect
management’s beliefs with respect to future events and are based on information currently available to
management on the date such statements were made. Many factors could cause Sernova’s actual results,
performances or achievements to not be as anticipated, estimated or intended or to differ materially from
those expressed or implied by the forward-looking statements contained in this news release. Such factors
could include, but are not limited to, the company’s ability to secure additional financing and licensing
arrangements on reasonable terms, or at all; ability to conduct all required preclinical and clinical studies for
the company’s Cell Pouch System and or related technologies, including the timing and results of those
trials; ability to obtain all necessary regulatory approvals, or on a timely basis; ability to in-license additional
complementary technologies; ability to execute its business strategy and successfully compete in the
market; and the inherent risks associated with the development of biotechnology combination products
generally. Many of the factors are beyond our control, including those caused by, related to, or impacted by
the novel coronavirus pandemic. Investors should consult the company’s quarterly and annual filings
available on www.sedarplus.ca for additional information on risks and uncertainties relating to the forward-
looking statements. Sernova expressly disclaims any intention or obligation to update or revise any forward-
looking statements, whether as a result of new information, future events or otherwise.
Subscribe to our Newsletter Abonnez-vous à notre newsletter
By subscribing to our Newsletter you will receive Sernova’s press releases and news as they develop by email. We do not send direct mail, customized online advertising, and we do not share your personal information. We respect your privacy. En vous abonnant à notre newsletter, vous recevrez par e-mail les communiqués de presse et les actualités de Sernova au fur et à mesure de leur développement. Nous n'envoyons pas de publipostage, de publicité en ligne personnalisée et nous ne partageons pas vos informations personnelles. Nous respectons votre vie privée.
Please read our Pour plus d’informations, veuillez lire notre Privacy policypolitique de confidentialité.








