Sernova Commercial Sernova Commercial
About Sernova, Corp. À propos de Sernova, Corp.
Sernova is committed to the development and clinical advancement of its products for metabolic, hematological and other chronic diseases using therapeutic cells transplanted into a patented implanted medical device, which forms an organ-like environment promoting long-term function and survival of the therapeutic cells. Sernova s'engage dans le développement et l'avancement clinique de ses produits contre les maladies métaboliques, hématologiques et autres maladies chroniques utilisant des cellules thérapeutiques transplantées dans un dispositif médical implanté breveté, qui forme un environnement semblable à un organe favorisant la fonction et la survie à long terme des cellules thérapeutiques.
The company’s management believes in building strong and long-lasting collaborations and partnerships that would lead to the rapid advancement of Sernova’s portfolio of products into the market, improving global health and bringing value to patients and society, in concert with our clinical development programs. La direction de la société croit à la mise en place de collaborations et de partenariats solides et durables qui permettraient au portefeuille de produits de Sernova de progresser rapidement sur le marché, d’améliorer la santé mondiale et d’apporter une valeur ajoutée aux patients et à la société, de concert avec nos programmes de développement clinique.
Indications: Les indications:
Diabetes Diabète
Haemophilia A Hémophilie A
Thyroid disease Maladie thyroïdienne
Clinical studies: Etudes cliniques:
Diabetes US phase I/II clinical study cleared by the FDA Etude clinique de phase I / II sur le diabète, approuvée par la FDA
First-in-human study in Diabetic subjects with hypoglycemia unawareness Première étude chez l'homme chez des sujets diabétiques peu conscients de l'hypoglycémie
Cell Pouch System™
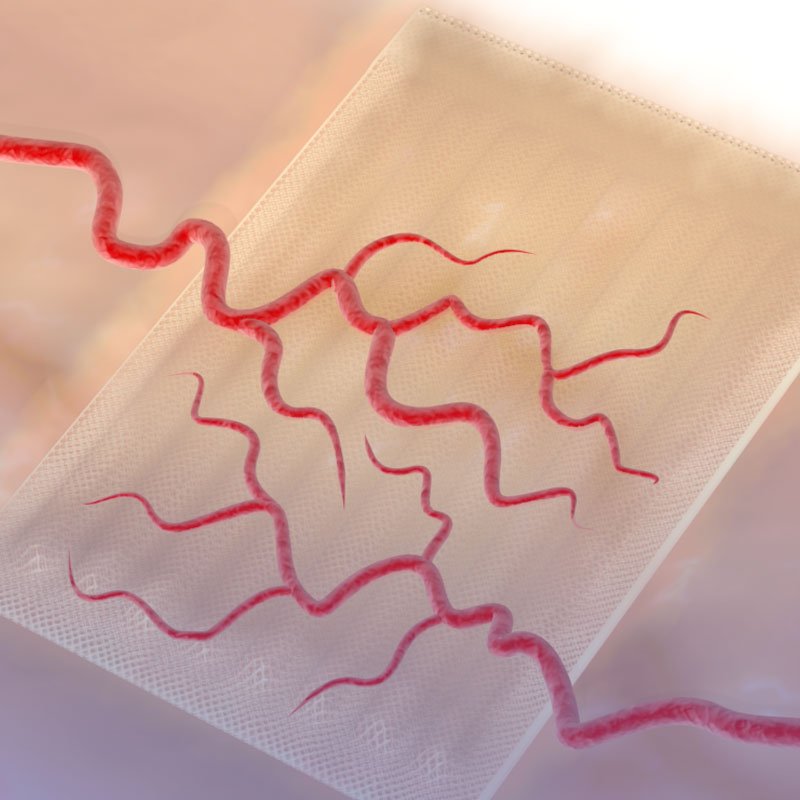
Sernova’s Cell Pouch System™ is a novel implantable and scalable medical device which forms a highly vascularized organ-like environment in the body for the housing, function and long-term survival of therapeutic cells. These therapeutic cells release necessary proteins or hormones missing from the body to treat chronic diseases as an alternative to daily administration of drugs. Le Cell Pouch System™ est un nouveau dispositif médical préalablement implanté et formant un environnement naturel hautement vascularisé afin d’y loger des cellules thérapeutiques, favorisant leur bon fonctionnement et leur survie dans le corps. Ces cellules thérapeutiques libèrent les protéines ou les hormones nécessaires pour traiter les maladies chroniques comme alternative a l’administration quotidienne de médicaments.

Immune Protection Protection immunitaire
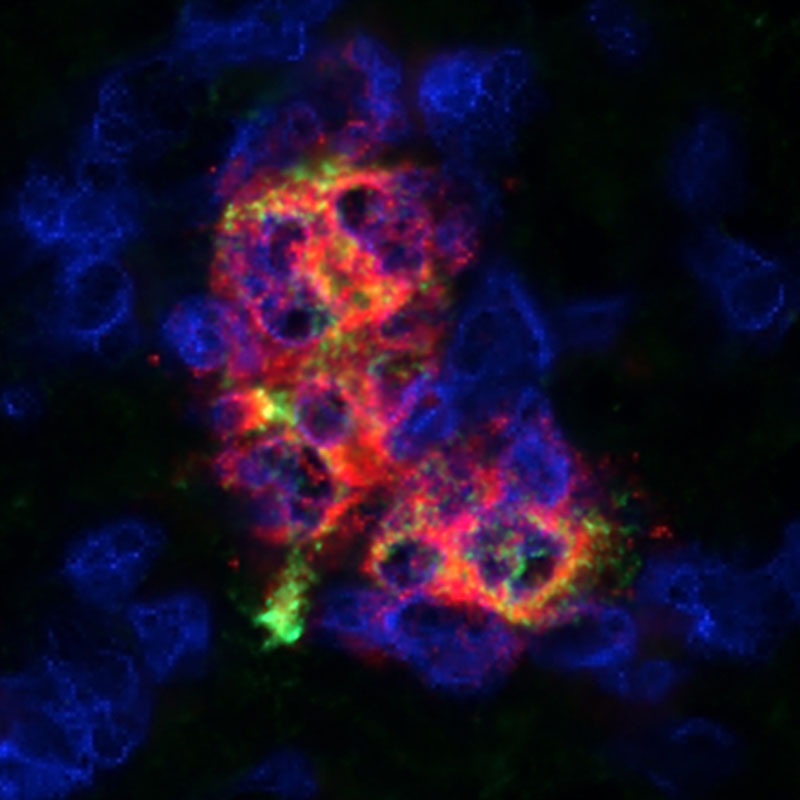
We have shown that cells can be protected using medications that prevent immune system attack within the Cell Pouch™. Nous avons montré que les cellules peuvent être protégées en utilisant des médicaments qui empêchent les attaques du système immunitaire dans la Cell Pouch™.
Microencapsulation technologies house cells within the Cell Pouch chambers and protect the cells from immune system attack. Les technologies de microencapsulation hébergent les cellules dans les chambres à cellules et les protègent des attaques du système immunitaire.
Technologies are in development to make transplanted cells unrecognizable to the immune system. Des technologies sont en cours de développement pour rendre les cellules greffées méconnaissables du système immunitaire.
Sernova’s Cell Pouch™, combined with immune protected therapeutic cells, offers protection from immune system attack creating an effective, safe, long-term and convenient therapeutic option for patients with chronic diseases who seek to improve their quality of life. Le Cell Pouch™ de Sernova, associé à des cellules immunitaires protégées, offre une protection contre le système immunitaire avec une option thérapeutique efficace, sûre, à long terme et pratique pour les patients atteints de maladies chroniques qui cherchent à améliorer leur qualité de vie.

Featured News Nouvelles en vedette
Sernova Business Update
Marek Sutherland of CTV News London - Cure for type one diabetes getting closer, London company says
Noah Stansfield of CGT Live - Patients With T1D Achieve Insulin Independence Following Implantation of Cell Pouch System and Islet Transplant
Sean Whooley and Danielle Kirsh of Fast Five- Teleflex has a Class I recall, Boston Scientific appoints two new board directors (Sernova discussed in the podcast recording from 1:00-2:58 time marks)
Lei Lei Wu of EndPoints News - Sernova says five diabetes patients have now been taken off insulin after 'cell pouch' therapy
Shane Whooley of MassDevice - Sernova reports positive interim data for Cell Pouch System
News Releases Communiqués de presse
Jonathan Rigby Appointed as Executive Chairman for Sernova
Sernova Announces Additional Patient Insulin Independent and Cohort 2 Enrollment Complete in Ongoing Phase I/II Clinical Trial for Treatment of Type 1
Sernova Announces Marketed Public Offering of Units
Sernova Appoints Jonathan Rigby to its Board of Directors
Updates Mises à jour
Sernova KOL Event on Thyroid Disease with Dr. Sam Wiseman
Watch now!
Visionner maintenant!
Events Événements
Sernova AGM - Annual General Meeting Sernova AGM - Annual General Meeting
2024 Bloom Burton & Co. Healthcare Investor Conference 2024 Bloom Burton & Co. Healthcare Investor Conference
Oppenheimer 34th Annual Healthcare Medtech & Services Conference Sernova Presentation at 1:20-1:50pm EST. Oppenheimer 34e conférence annuelle sur les technologies médicales et les services de santé Présentation Sernova à 13h20-13h50 HNE.
Invest with Sernova Investir dans Sernova
If you are a shareholder, investor, broker, analyst, journalist, investment advisor, or looking to develop business opportunities, please feel free to contact us by email or telephone. Si vous êtes actionnaire, investisseur, courtier, analyste, journaliste, conseiller en placement ou souhaitez développer des opportunités d’affaires, n'hésitez pas à nous contacter par email ou par téléphone.
Sernova is a regenerative medicine company developing therapeutic technologies with multibillion-dollar market potential for each of its clinical indications. Sernova est une société de médecine régénérative développant des technologies thérapeutiques offrant un potentiel de marché de plusieurs milliards de dollars pour chacune de ses indications cliniques.
Sernova is a Collaborative Team Sernova, c’est aussi les collaborations
We believe in advancing our clinical programs and building strong and long-lasting collaborations and partnerships that will lead to the rapid advancement of Sernova’s portfolio of products into the market, improving global health and bringing value to patients and society. Nous croyons en la promotion de nos programmes cliniques et en la mise en place de collaborations et de partenariats solides et durables qui permettront au portefeuille de produits de Sernova de progresser rapidement sur le marché, d'améliorer la santé mondiale et d'apporter de la valeur aux patients et à la société.
Privacy PolicyPolitique de confidentialité
Updated July 6, 2018
Please read this Policy carefully along with our Legal Notice that describes our Terms of Use for the Website.
By accessing www.sernova.com (the “Website”) you hereby agree with the practices described in this Privacy Policy (the “Policy”)
This Policy applies to all information gathered through the Website and/or any related marketing technique or events.
Information Collection
The information collected is limited to the information that you decide to share with us through the News Dispatch Service, when participating at event or activities or in the general course of business by expressing an interest in obtaining information about Sernova Corp. and our products, such as name, email, phone number, and similar contact information. This information is stored through MailChimp (please refer to MailChimp Privacy Policy at https://mailchimp.com/legal/privacy/).
Information Sharing
Sernova Corp. is the sole owner of any information collected on the Website. We do not sell, share or rent this information to others.
Traffic and Automatic Information Collection
Sernova Corp. maintains log files of the traffic on www.sernova.com. This information is not linked to any personal information that you have provided us. Logs are used to manage traffic, identify content accessed, and IT requirements. Information logged and automatically collected includes without being limited to IP addresses and browser types. This information does not reveal your specific identity.
Cookies
Cookies can be used to provide you with a more personalized experience. The Website may use cookies to make that experience more companionable when you return to the Website. You have the option at all time to decline the use of cookies. If you choose to do so, you may not be able to fully use all features of the Website. You can also delete cookie files at all time from your computer. Those cookies may include first-party cookies (such as the Google Analytics cookies).
Updates
This Policy is a living document and may be amended or updated from time to time without further notice. We encourage you to review the Policy periodically.
Contact
If you have any questions or comments about our policy, you can email us at info@sernova.com or by phone at 1(877) 299-4603 or by mail at
Sernova Corp.
700 Collip Circle, Suite 114
London, ON Canada N6G 4X8
Mis à jour le 6 juillet 2018
Veuillez lire attentivement cette politique ainsi que notre avis juridique qui décrit nos conditions d'utilisation du site Web.
En accédant à www.sernova.com (le «site Web»), vous acceptez les pratiques décrites dans la présente politique de confidentialité (la «politique»).
Cette politique s'applique à toutes les informations collectées via le site Web et / ou toute technique ou événement marketing associé.
Collecte d'informations
Les informations collectées se limitent aux informations que vous décidez de partager avec nous par le biais du service d’expédition de nouvelles, lorsque vous participez à un événement ou à des activités ou que vous vous intéressez à obtenir des informations sur Sernova Corp. comme nom, email, numéro de téléphone et informations de contact similaires. Ces informations sont stockées via MailChimp (veuillez vous reporter aux règles de confidentialité de MailChimp sur https://mailchimp.com/legal/privacy/).
Partage d'information
Sernova Corp. est l'unique propriétaire de toute information collectée sur le site Web. Nous ne vendons pas, ne partageons pas ou ne louons pas ces informations à des tiers.
Collecte d'informations routières et automatiques
Sernova Corp. gère les fichiers journaux du trafic sur www.sernova.com. Ces informations ne sont liées à aucune information personnelle que vous nous avez fournie. Les journaux sont utilisés pour gérer le trafic, identifier le contenu accédé et les besoins informatiques. Les informations consignées et collectées automatiquement ne sont pas limitées aux adresses IP et aux types de navigateur. Cette information ne révèle pas votre identité spécifique.
Cookies
Les cookies peuvent être utilisés pour vous offrir une expérience plus personnalisée. Le site Web peut utiliser des cookies pour rendre cette expérience plus conviviale lorsque vous revenez sur le site Web. Vous avez la possibilité à tout moment de refuser l'utilisation de cookies. Si vous choisissez de le faire, vous ne pourrez peut-être pas utiliser toutes les fonctionnalités du site Web. Vous pouvez également supprimer des fichiers de cookies à tout moment depuis votre ordinateur. Ces cookies peuvent inclure des cookies de première partie (tels que les cookies de Google Analytics).
Mises à jour
Cette politique est un document évolutif et peut être modifié ou mis à jour de temps à autre sans préavis. Nous vous encourageons à consulter la politique périodiquement.
Contact
Si vous avez des questions ou des commentaires sur notre politique, vous pouvez nous envoyer un courriel à info@sernova.com ou par téléphone au 1 (877) 299-4603 ou par courrier à
Sernova Corp.
700 Collip Circle, Suite 114
London, ON Canada N6G 4X8
Press Release Communiqué de presse - Juillet 15, 2024 15 Juillet, 2024
Jonathan Rigby Appointed as Executive Chairman for Sernova
LONDON, Ontario; BOSTON, Massachusetts – July 15, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative
medicine cell therapies for treatment of chronic diseases such as Type 1 Diabetes, today announced that the
Board of Directors has appointed Jonathan Rigby, who previously joined as a Director in May, as Executive
Chairman effective immediately. Brett Whalen, who was previously Chairman, has resigned from the Board.
These changes continue carefully curated management and Board transitions aimed at positioning Sernova to
enter the next phase of the Company’s growth and evolution.
As Executive Chairman Mr. Rigby will focus on partnering with company leadership on capital markets efforts
for Sernova as well as leadership of the Board. Cynthia Pussinen, the Company’s Chief Executive Officer, will
continue in her role as originally defined managing Sernova’s day to day business strategy and operations; she
will continue to report to the Board.
“We welcome Jonathan to his expanded role as Executive Chairman to increase our focus on capital markets
where he has a demonstrated track record of success through multiple capital raises, Nasdaq listings and
successful exits of several biotech companies,” said James Parsons, Director of Sernova. “The Board fully
supports the leadership team and Jonathan will further augment our overall Company strength. We are grateful
to Brett for his Chairmanship.”
“I am delighted to serve the Company and its shareholders as Executive Chairman with passion and
enthusiasm. As a sufferer myself, I will do all I can to help the Company give lives back to patients suffering
from diseases such as Type 1 Diabetes” said Jonathan Rigby, Executive Chairman. “I have financed and grown
multiple companies through to exits and my goal is quite clear, I will work tirelessly with the team to do the
same for Sernova and its shareholders.
Mr. Rigby has held several CEO roles and currently serves on the Board of Directors of cancer therapy company
Oncolytics Biotech Inc. (NASDAQ: ONCY) and IM Therapeutics and was formerly on the board of Xeris
Pharmaceuticals (NASDAQ: XERS). Mr. Rigby holds a degree in Biological Sciences from the University of
Sheffield, UK, and has a Master of Business Administration degree from the University of Portsmouth, UK.
Sernova Corp continues to collaborate closely with leading academic, pharmaceutical and clinical institutions to
expand the scope and impact of its technology. The company anticipates further advancements as it progresses
through additional cohorts and trials, with the ultimate goal of offering a scalable solution for insulin-dependent
diabetes plus other chronic diseases.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in the
bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to
be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - Juin 25, 2024 25 Juin, 2024
Sernova Announces Additional Patient Insulin Independent and Cohort 2 Enrollment Complete in Ongoing Phase I/II Clinical Trial for Treatment of Type 1 Diabetes
Sernova Announces Additional Patient Insulin Independent and Cohort 2 Enrollment Complete in Ongoing
Phase I/II Clinical Trial for Treatment of Type 1 Diabetes
- To date Sernova reports 7 patients in its Phase I/II type 1 diabetes clinical trial achieved freedom from insulin
injections and demonstrate blood sugar control in the non-diabetic range (HbA1c <6.5%);
- 6 patients have reached between 5.5 and 50 months of sustained insulin independence and freedom from
severe hypoglycemic episodes;
- Completion of enrollment in Cohort 2
LONDON, Ontario; BOSTON, Massachusetts – June 25, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company, today announces developments in its Phase I/II
clinical trial investigating a novel treatment for Type 1 Diabetes (T1D). To date a total of 7 patients across
Cohorts 1 and 2 have achieved insulin independence following transplants of human donor islets via Sernova’s
proprietary Cell Pouch System™ and a marginal portal vein top up. Additionally, the study, which is being
conducted at the University of Chicago, completed full enrollment of the second cohort of patients with the last
patient being enrolled June 24, 2024.
At baseline, all patients in the study were dependent on multiple daily insulin injections with average HbA1c
levels (a measure of glucose control over the prior 2 to 3 months) greater than 6.5% across the study
population. In addition, prior to enrollment in the study, patients had a history of severe hypoglycemic events (a
potentially life-threatening drop in blood sugar leading to impaired cognition and consciousness) and
undetectable plasma levels of C-peptide (a marker of natural insulin production). Insulin independence in these
first 7 patients has been accompanied by freedom from severe hypoglycemic events, sustained HbA1c levels in
the non-diabetic range (HbA1c <6.5%), and persistent transplant-mediated insulin production measured by
plasma C-peptide.
The trial, which focuses on Sernova’s Cell Pouch technology, demonstrates that insulin independence and
additional clinically meaningful patient outcomes were achieved with lower than traditional islet masses infused
intra-portally, indicating an important contribution of the islet grafts in Cell Pouch to the observed favorable
blood glucose control. Sernova continues to be the first and only company to announce positive patient
outcomes in a clinical trial for T1D that combines donor islets transplanted via an implantable delivery vehicle
that also has full payload containment and retrievability capabilities should it be required, as evidenced by the
first patient in Cohort 2. Interim data from this study indicates that Cell Pouch is generally safe and well
tolerated and, when transplanted with donor islets, contributes to the reversal of T1D, potentially minimizing
the long-term comorbidities that include heart and kidney disease, blindness and amputation. The Company
anticipates announcing additional trial results for the remaining 5 patients in Cohort 2, later in the year.
Cynthia Pussinen, CEO of Sernova, expressed "we are excited with and encouraged by the impressive progress
of our ongoing Phase I/II clinical trial with donor islets and Cell Pouch. The ability to achieve sustained insulin
independence, HbA1c levels in the non-diabetic range and reductions in severe hypoglycemic events in
patients, using a retrievable payload device, signifies a transformative step forward in the treatment – and
essentially functional cure - of Type 1 Diabetes. Sernova remains committed to advancing the potential of our
Cell Pouch technology to significantly improve the lives of patients and their families worldwide.”
Sernova Corp continues to collaborate closely with leading academic, pharmaceutical and clinical institutions
to expand the scope and impact of its technology. The company anticipates further advancements as it
progresses through additional cohorts and trials, with the goal of offering a scalable solution for insulin-
dependent diabetes plus other chronic diseases.
About Sernova
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of
insulin producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2).
Sernova’s development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid
disease resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
About Sernova’s Cell Pouch for use with Cell Therapeutics
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing
longterm survival and function of therapeutic cells that release essential factors that are absent or deficient in
the bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its
potential to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of
Chicago. The Cell Pouch technology integrates with the body's natural biological processes, creating a
biocompatible environment for the delivery of therapeutic cells previously missing from the body including
cells, hormones, blood factors and tissues. This innovative approach aims to provide a durable and sustainable
treatment option for Sernova’s lighthouse program to treat individuals living with T1D, with additional future
therapeutic programs to follow for hypothyroidism and hemophilia A.
For further information, please contact:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
Forward Looking Information:
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - Juin 11, 2024 11 Juin, 2024
Sernova Announces Marketed Public Offering of Units
NEWS RELEASE
Sernova Announces Marketed Public Offering of Units
NOT FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR FOR DISSEMINATION IN THE
UNITED STATES
LONDON, Ontario; BOSTON, Massachusetts – June 6, 2024, Sernova Corp. (“Sernova” or the “Company”)
(TSX:SVA) (OTCQB:SEOVF) (FSE/XETRA:PSH) is pleased to announce that it has filed a preliminary short
form prospectus in connection with a best efforts marketed public offering (the “Offering”) of units of the
Company (the “Units”). The Offering is being led by Stifel Nicolaus Canada Inc., as lead agent (the “Lead
Agent”) and joint bookrunner with Leede Jones Gable Inc., on behalf of a syndicate of agents that includes
Ventum Financial Corp., Raymond James, Research Capital Corporation and Roth Canada, Inc. (together
with the Lead Agent, the “Agents”) and is for minimum gross proceeds of $6,500,000 and up to maximum
gross proceeds of $10,000,000. Each Unit will be offered at a price of $0.33 per Unit (the “Offering Price”),
and shall consist of one (1) common share of the Company (each, a “Common Share”) and one (1) Common
Share purchase warrant (a “Warrant”). Each Warrant will entitle the holder to purchase one Common Share
(each, a “Warrant Share”) at an exercise price of $0.40 per Warrant Share for 36 months. It is expected that
a definitive agency agreement will be entered into between the Company and the Agents following the
successful marketing of the Offering.
The Company has agreed to grant the Agents an option (the “Over-Allotment Option”) to sell up to an
additional number of Units at the Offering Price as is equal to 15% of the number of Units issued pursuant to
the Offering, exercisable in whole or in part, at any time and from time to time on or prior to the date that is
30 days following the Closing Date (as defined below) to cover over-allotments, if any, and for market
stabilization purposes. The Over-Allotment Option shall be exercisable for any number of Units, Common
Shares, Warrants, or any combination thereof at a price equal to the Offering Price for a Unit and a price to
be agreed upon for the Common Shares and Warrants.
The Company plans to use the net proceeds from the offering to expand enrollment in the Company’s phase
I/II Human Donor Islet clinical study, to support the Company’s R&D activities and for general corporate
purposes. In addition Sernova would like to announce that Evotec SE, its largest shareholder and
collaboration partner, will participate in the offering.
The Offering is scheduled to close on or about June 20, 2024 (the “Closing Date”), and is subject to certain
conditions including, but not limited to, the receipt of all necessary approvals, including the approval of the
Toronto Stock Exchange (the “Exchange”).
A preliminary short form prospectus containing important information relating to the Offering has been filed
with the securities regulatory authorities in each of the provinces in Canada, other than the Province of
Québec, and is still subject to completion or amendment. The preliminary short form prospectus is available
via SEDAR+ at www.sedarplus.ca. Alternatively, the Company, any Agent or any dealer participating in the
Offering will arrange to send you the preliminary short form prospectus upon request from the Lead Agent at
ecmcanada@stifel.com.
This press release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities
in the United States. The securities have not been and will not be registered under the United States
Securities Act of 1933, as amended (the “U.S. Securities Act”), or any U.S. state securities laws and may not
be offered or sold to or for the account or benefit of persons in the “United States” or “U.S. persons” (as
such terms are defined in Regulation S under the U.S. Securities Act) unless registered under the U.S.
Securities Act and applicable state securities laws or an exemption from such registration is available.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with
immune protected therapeutic cells.
On implantation, the Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in the
bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential
to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of
Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
For further information, please contact:
Christopher Barnes
VP, Investor Relations Sernova Corp.
christopher.barnes@sernova.com
519-902-7923 www.sernova.com
Press Release Communiqué de presse - Mai 28, 2024 28 Mai, 2024
Sernova Appoints Jonathan Rigby to its Board of Directors
Sernova Appoints Jonathan Rigby to its Board of Directors
LONDON, Ontario; BOSTON, Massachusetts – May 28, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative
medicine cell therapies for treatment of chronic diseases, announces today that Jonathan Rigby will join
Sernova’s Board of Directors effective immediately.
“We are excited to welcome Jonathan to Sernova’s Board of Directors. With more than 30 years of diverse
experience across the biopharmaceutical landscape, Jonathan brings a track record of substantial
contributions across our sector as a biotech CEO and Board member. He will both complement and
augment the expertise and composition of our current Board,” said Cynthia Pussinen, Chief Executive
Officer of Sernova. “We welcome Jonathan’s passion for improving the lives of patients and his guidance on
accelerating delivery of our development programs.”
Mr. Rigby has a breadth of industry knowledge and productive experience having served as President and
CEO of Revolo Biotherapeutics where he took the company through multiple financings and two Phase 2
clinical trials, as well as serving as Chairman and Chief Business Officer of BIOS Acquisition Corporation
(NASDAQ: BIOS), with an oversold IPO. Mr. Rigby served as the CEO for SteadyMed Therapeutics (NASDAQ:
STDY), which he listed on Nasdaq prior to its acquisition by United Therapeutics (NASDAQ: UTHR), and was
co-founder of Zogenix (NASDAQ:ZGNX), which was also acquired by UCB (Euronext: UCB) after completing
a NASDAQ listing.
In addition, Mr. Rigby currently serves on the Board of Directors of cancer therapy company Oncolytics
Biotech Inc. (NASDAQ: ONCY) and IM Therapeutics which is developing therapies for Type 1 diabetes (T1D).
He is a past Board Member of Thermalin Inc., which engineered new forms of insulin to improve patient lives
and outcomes for the treatment of T1D and was formerly on the board of Xeris Pharmaceuticals (NASDAQ:
XERS) which developed and commercialised a therapy to treat T1D severe hypoglycemia. Mr. Rigby holds a
degree in Biological Sciences from the University of Sheffield, UK, and has a Master of Business
Administration degree from the University of Portsmouth, UK.
“As an individual with T1D, I am acutely aware of the challenges of living with this life changing condition and
the need to effectively cure the disease. Given Sernova’s vision of a future where chronic conditions are no
longer insurmountable obstacles, and their foundational work on delivering a ‘functional cure’ for T1D, I am
especially excited to join the Board and work hard to progress the company forward. Sernova represents a
new era of development for cell therapy treatments and I look forward to working with the Board and the
leadership team in advancing Sernova’s groundbreaking technology and therapies,” said Mr. Rigby.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with
immune protected therapeutic cells. On implantation, The Cell Pouch forms a natural vascularized tissue
environment in the body for long-term survival and function of therapeutic cells that release essential
factors that are absent or deficient in the bodies of patients with certain chronic diseases. Sernova’s Cell
Pouch System has demonstrated its potential to be a ‘functional cure’ for people with T1D in an ongoing
Phase 1/2 clinical study at the University of Chicago. Sernova partnered with Evotec to develop an
implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet replacement therapy. This
partnership provides Sernova a potentially unlimited supply of insulin-producing cells to treat millions of
patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s development pipeline that uses its
Cell Pouch System also includes: a cell therapy for hypothyroid disease resulting from thyroid gland removal
and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible,
but not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates",
"projects", "potential for" and similar expressions, or that events or conditions "will", "would", "may",
"could" or "should" occur are used to identify forward-looking statements. These statements reflect
management’s beliefs with respect to future events and are based on information currently available to
management on the date such statements were made. Many factors could cause Sernova’s actual results,
performances or achievements to not be as anticipated, estimated or intended or to differ materially from
those expressed or implied by the forward-looking statements contained in this news release. Such factors
could include, but are not limited to, the company’s ability to secure additional financing and licensing
arrangements on reasonable terms, or at all; ability to conduct all required preclinical and clinical studies for
the company’s Cell Pouch System and or related technologies, including the timing and results of those
trials; ability to obtain all necessary regulatory approvals, or on a timely basis; ability to in-license additional
complementary technologies; ability to execute its business strategy and successfully compete in the
market; and the inherent risks associated with the development of biotechnology combination products
generally. Many of the factors are beyond our control, including those caused by, related to, or impacted by
the novel coronavirus pandemic. Investors should consult the company’s quarterly and annual filings
available on www.sedarplus.ca for additional information on risks and uncertainties relating to the forward-
looking statements. Sernova expressly disclaims any intention or obligation to update or revise any forward-
looking statements, whether as a result of new information, future events or otherwise.
Press Release Communiqué de presse - Mai 01, 2024 1 Mai, 2024
Sernova Announces AGM Voting Results
SERNOVA ANNOUNCES AGM VOTING RESULTS
LONDON, Ontario; BOSTON, Massachusetts – May 1, 2024, Sernova Corp. (“Sernova” or the “Corporation”)
(TSX:SVA) (OTCQB:SEOVF) (FSE/XETRA:PSH) a clinical-stage biotechnology company focused on the
development of regenerative medicine cell therapies for treatment of chronic diseases, is pleased to announce
results from its Annual General Meeting of Shareholders (the “AGM”) held virtually via live audio webcast, on
April 30, 2024. At the Meeting, a total of 88,316,160 common shares were voted, representing 29.11% of the
votes attached to all outstanding common shares as of the record date. The voting results were as follows:
| Director | % of Votes For | % of Votes Against |
| Cynthia Pussinen | 95.62% | 4.38% |
| James T. Parsons | 98.63% | 1.37% |
| Dr. Steven Sangha | 96.43% | 3.57% |
| Brett A. Whalen | 73.91% | 26.09% |
Accordingly, Cynthia Pussinen, James T. Parsons, Dr. Steven Sangha and Brett A. Whalen were elected to the
board of directors for the ensuing year. In addition, Bernd Muehlenweg was appointed to the board of directors
following the AGM. Bertram von Plettenberg resigned from the board of directors prior to the AGM and did not
stand for election at the AGM.
Shareholders also approved (with 99.79% of the votes approving) the re-appointment of KPMG LLP, Chartered
Professional Accountants as the Company’s auditor for the ensuing year, and approved (with 82.57% of the
votes approving) the amendments to the Option Plan and DSU Plan and the increase in the maximum number of
Common Shares reserved for issuance.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in the
bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to
be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable o-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
christopher.barnes@sernova.com
519-902-7923
www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to dier materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - Avril 25, 2024 25 Avril, 2024
Sernova Provides Organizational Update
Sernova Provides Organizational Update
• Dr. Philip Toleikis to retire as of April 30, 2024
• Cost savings from restructuring and strategic transformation anticipated to extend runway
• Board rebuild underway
LONDON, Ontario; BOSTON, Massachusetts – April 25, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative
medicine cell therapies for treatment of chronic diseases, today announced the retirement of Chief Technology
Officer (CTO), Dr. Philip Toleikis along with progress on strategic transformation efforts including a
restructuring of operations and a workforce reduction of approximately 35%.
Commenting on his retirement, Dr. Toleikis said, “It has been a tremendous honor and privilege to serve
Sernova since 2009, initially as the President, CEO and director, and more recently as its CTO. Significant
progress has been made and there is exciting work ahead that I am confident will be realized by the team,
under the guidance of our CEO, Ms. Pussinen.” Dr. Toleikis further states, “I am pleased to provide continued
support to Sernova as a consultant. I am proud of the Company’s advancements towards redefining the way
chronic diseases will be treated using a cell therapy-based approach while importantly contributing to
improving the lives of those living with chronic diseases.”
“Philip’s contributions to Sernova’s research and development, and fund-raising efforts have been foundational
to the Company’s evolution into a next-generation regenerative medicine company,” said Brett Whalen,
Chairman of the Sernova Board. “He will leave a company which is well-positioned to foster the next wave of
innovations in stem cell therapies. On behalf of the Board of Directors and the entire company, I would like to
thank Dr. Philip Toleikis for his contributions and unwavering dedication to the Company. We wish him all the
best for his future.”
As communicated in early April, following a review of the company’s therapeutic pipeline and emerging
opportunities for its Cell Pouch system platform technologies, Sernova confirmed key priorities including its
lighthouse program in insulin dependent Type 1 Diabetes plus its intention to advance an IND filing for its post-
operative hypothyroidism program. “The pipeline and platform review and associated strategy refresh
highlighted several ways by which to optimize financial resources, extending the Company’s cash runway. We
continue to seek ways to raise additional capital to strengthen our financial foundation,” said Cynthia Pussinen,
Chief Executive Officer of Sernova. “In connection with the strategic transformation, we will pause on any new
investments into the conformal coating program to reallocate funds. In parallel, we continue to evaluate
alternative approaches to obviate the need for immunosuppressive regimens for our allogeneic therapies.”
Sernova has implemented a plan to fortify the balance sheet and cash position, including a workforce
restructuring, representing a key step towards streamlining the organization while ensuring the ability to secure
core competencies needed to drive further progress in key clinical and pre-clinical assets. “Decisions to scale
back or to rebalance headcount are extremely difficult. I want to express my heartfelt appreciation and
gratitude to those impacted for their invaluable contributions, dedication to helping patients and impact to
furthering the Company’s vision of a future where chronic conditions are no longer insurmountable obstacles”,
said Ms. Pussinen.
The Company confirms the completion and closure of all internal investigation efforts, previously announced
with respect to its former CFO and potentially a second employee of the Company. No new findings were
revealed and there will be no further action on the matter. The investigation confirmed that there have been no
securities violations and that findings bore no material impact to financial statements and operations.
Lastly, Sernova Director Mr. Bertram von Plettenberg has retired from the Company’s Board, effective April
23rd. “The Board and Management sincerely thank Mr. von Plettenberg for his past year of service to the
Company, and wish him well in future endeavors,” said Ms. Pussinen. “The evolution and composition of the
Board will continue, as we look to enhance our life sciences industry specific strength, to better serve our
patients and shareholders.”
ABOUT SERNOVA’S ANNUAL GENERAL MEETING
Sernova’s Annual General Meeting will be held on Tuesday April 30, 2024 starting at 1:00 PM ET. The meeting
will be conducted virtually, via live webcast and accessible online at https://virtualmeetings.tsxtrust.com/1571.
Please note that this site may not be fully accessible on all internet browsers, and it is advisable to use a
browser other than Internet Explorer for optimal experience. If you are unable to join the meeting through your
usual web browser, we suggest trying an alternate browser.
For investors who wish to submit questions via the TSX Trust portal voting control numbers will be needed. For
attendees who would simply like to listen to the meeting conduct, you can register as a guest.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells.
On implantation, The Cell Pouch forms a natural, vascularized tissue environment in the body allowing long-
term survival and function of therapeutic cells that release essential factors that are absent or deficient in the
bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to
be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells)
based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-
producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s
development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease
resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - Avril 22, 2024 22 Avril, 2024
Sernova Welcomes Dr. Bernd Muehlenweg as Evotec’s Nominee for its Board of Directors
Dr. Daniel Mahony to step down to pursue new commitment
LONDON, Ontario; BOSTON, Massachusetts – April 22, 2024 – Sernova Corp. (TSX:SVA)
(OTCQB:SEOVF) (FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the
development of regenerative medicine cell therapies for treatment of chronic diseases, announces
today that Dr. Bernd Muehlenweg, Senior Vice President of Global Business Development at Evotec
SE (Frankfurt Stock Exchange: EVT, MDAX/TecDAX, ISIN: DE0005664809; NASDAQ: EVO), has been
appointed to its Board of Directors. Evotec recently shared that due to the impending departure of Dr. Daniel
Mahony, its current Sernova Board delegate, who is leaving to pursue a new professional
undertaking, they would be nominating a new Board representative. With heartfelt gratitude, we
wish Dan all the very best in his future endeavors.
Dr. Muehlenweg will join Sernova’s Board of Directors effective immediately. Sernova expects the
brief period of overlap with Dr. Daniel Mahony will assist in the transition bringing Dr. Muehlenweg
rapidly up to speed on Sernova’s operational and strategic matters.
Bernd Muehlenweg's work experience includes various senior leadership positions in the biotech
and pharmaceutical industry with a concentration on business development and alliance
management roles. At Evotec, he leads the company's partnering and out-licensing efforts in their
focus areas including iPSC-based Cell Therapy, Panomics-driven drug discovery, Oncology,
Immunology & Inflammation, Infectious Diseases, Predictive Safety and Enabling Technologies.
Prior to joining Evotec, he held the position of Chief Business Officer and served on the Executive
Board of Nanobiotix, a French clinical stage oncology company. Bernd co-founded Panoptes
Pharma GmbH, an Austrian biotech company focused on developing therapies for eye diseases,
which was later acquired by Eyegate Pharmaceuticals. Additionally, at Wilex AG, he played a key role
in the company's growth and expansion. He began his career as a Group Leader at the Technical
University of Munich, Germany.
Bernd Muehlenweg graduated with a Ph.D. from the oncology research group at the Department of
Gynecology at the Technical University of Munich in 2000. He further attended management classes
at the Switzerland based St. Galler Business School in 2006.
“Sernova’s Cell Pouch and Evotec’s iPSC derived islet like clusters are a powerful combination,
offering a potential functional cure for type 1 diabetes (T1D) in the not-too-distant future. My
personal goal is to foster sustainable, fruitful and durable partnerships to advance scientific
innovations into approved treatments. I have tremendous confidence that the strategic
collaboration between Evotec and Sernova will provide a strong foundation to potentially achieve
this objective” said Bernd Muehlenweg, Sernova’s new Board Director.
“At Sernova, we will continue to pursue opportunities to improve the lives of patients with unmet
needs while building long-term value for shareholders in multiple ways. I cannot stress enough the
importance of the Cell Pouch System as the anchor for our ongoing T1D trial. In addition to its longterm
payload survival, containment and retrievability characteristics, we believe that the Cell Pouch
is a key differentiator in delivering clinically meaningful outcomes, including insulin independence
and normalized HbA1c counts amongst others, to several patients in our ongoing Phase 1/2 trial. We
are pleased to welcome Bernd as he joins us on our mission to build a future where chronic
conditions are no longer insurmountable obstacles.” said Cynthia Pussinen, CEO of Sernova.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell
technologies for chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood
disorders that include hemophilia A. Sernova is currently focused on developing a ‘functional cure’
for insulin-dependent diabetes with its lead technology, the Cell Pouch System, a novel implantable
and scalable medical device with immune protected therapeutic cells.
On implantation, The Cell Pouch forms a natural vascularized tissue environment in the body for
long-term survival and function of therapeutic cells that release essential factors that are absent or
deficient in the bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has
demonstrated its potential to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2
clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent
stem cells) based islet replacement therapy. This partnership provides Sernova a potentially
unlimited supply of insulin-producing cells to treat millions of patients with insulin-dependent
diabetes (type 1 and type 2). Sernova’s development pipeline that uses its Cell Pouch System also
includes: a cell therapy for hypothyroid disease resulting from thyroid gland removal and an ex vivo
lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
Tel: +1 519-902-7923
Email: christopher.barnes@sernova.com
Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may
constitute “forward-looking statements” that involve various risks, uncertainties, and assumptions,
including, without limitation, statements regarding the prospects, plans, and objectives of the
company. Wherever possible, but not always, words such as "expects", "plans", "anticipates",
"believes", "intends", "estimates", "projects", "potential for" and similar expressions, or that events
or conditions "will", "would", "may", "could" or "should" occur are used to identify forward-looking
statements. These statements reflect management’s beliefs with respect to future events and are
based on information currently available to management on the date such statements were made.
Many factors could cause Sernova’s actual results, performances or achievements to not be as
anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not
limited to, the company’s ability to secure additional financing and licensing arrangements on
reasonable terms, or at all; ability to conduct all required preclinical and clinical studies for the
company’s Cell Pouch System and or related technologies, including the timing and results of those
trials; ability to obtain all necessary regulatory approvals, or on a timely basis; ability to in-license
additional complementary technologies; ability to execute its business strategy and successfully
compete in the market; and the inherent risks associated with the development of biotechnology
combination products generally. Many of the factors are beyond our control, including those caused
by, related to, or impacted by the novel coronavirus pandemic. Investors should consult the
company’s quarterly and annual filings available on www.sedarplus.ca for additional information on
risks and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any
intention or obligation to update or revise any forward-looking statements, whether as a result of
new information, future events or otherwise.
Press Release Communiqué de presse - Avril 02, 2024 2 Avril, 2024
Sernova Provides Positive Clinical and Platform Portfolio Update
Sernova Provides Positive Clinical and Platform Portfolio Update
LONDON, Ontario; BOSTON, Massachusetts – April 2, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF) (FSE/XETRA:PSH), a clinical-stage biotechnology company focused on the development of regenerative medicine cell therapies for treatment of chronic diseases, today provided a business update. Following a review of the company’s therapeutic pipeline and emerging opportunities for its Cell Pouch system platform technologies, Sernova confirms key priorities including its lighthouse program in insulin dependent Type 1 Diabetes plus its intention to advance an IND filing for its post-operative hypothyroidism program.
Data from a patient in Cohort 2 of the company’s lead clinical program for insulin dependent Type 1 diabetes (T1D) confirms histologic evidence of long-term (one year) robust survival of abundant human donor islets throughout the Cell Pouch. Additional Cohort 2 findings are specific to an advanced immunosuppression regimen planned for use in its upcoming Phase I/II trial with stem cell-derived islets under co-development with Evotec.
Cohort 2 patients treated with an advanced immunosuppression protocol avoided graft rejection and experienced minimal side eects in comparison to those patients observed in Cohort 1. None of the six patients in Cohort 2 treated with the advanced regimen have tested positive for donor specific antibodies (DSAs), a marker of graft rejection, in comparison to three of six patients who developed DSAs under the conventional immunosuppression regimen in Cohort 1. Ancillary medication, used in some Cohort 2 patients, demonstrated highly favorable graft survival and function for islets transplanted to the Cell Pouch and has been integrated into the updated regimen and implemented for all subsequent patient trial enrollments. The company anticipates reporting additional data from Cohort 2 of its ongoing Phase 1/2 clinical trial of its expanded 10-channel Cell Pouch during the second half of the year at major medical conferences. Sernova is pleased to report that this month marks the four-year anniversary of the first patient in Cohort 1 of this Phase 1/2 study who will celebrate insulin independence and normalized blood sugar levels, based on two transplants of human donor islets to the Cell Pouch plus a marginal portal vein top up.
“Based on the favorable results we are observing in ongoing pre-clinical studies, we have concluded that our hypothyroidism program represents another compelling opportunity by which to improve patients’ lives. We look forward to completing our pre-clinical work, engaging with regulatory agencies, and preparing for an IND filing later this year, with the goal of advancing a second indication into the clinic, further demonstrating the Cell Pouch as a drug delivery vehicle platform technology. Also of note, in addition to allowing for long term payload survival, our Cell Pouch has powerful containment and retrievability capabilities that we expect will have tremendous value for pharmaceutical companies looking to treat patients with cell therapies,” said Cynthia Pussinen, Chief Executive Oicer of Sernova.
“In parallel with these activities, and our ongoing hemophilia A work, we have identified several high value indications with unmet medical needs that could potentially benefit from our platform Cell Pouch technology, with an initial focus on endocrine disorders. In the coming months, we will be conducting commercial assessments to prioritize those areas where we can best extend our reach to more patients while creating enduring value for our shareholders. I am excited for what we are poised to achieve this year and look forward to providing further updates in the future,” Ms. Pussinen concluded.
ABOUT SERNOVA AND ITS CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes with its lead technology, the Cell Pouch System, a novel implantable and scalable medical device with immune protected therapeutic cells. On implantation, The Cell Pouch forms a natural vascularized tissue environment in the body for long-term survival and function of therapeutic cells that release essential factors that are absent or deficient in the bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated its potential to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University of Chicago.
Sernova partnered with Evotec to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-producing cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova’s development pipeline that uses its Cell Pouch System also includes: a cell therapy for hypothyroid disease resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
- Christopher Barnes
- VP, Investor Relations
- Sernova Corp.
- Tel: +1 519-902-7923
- Email: christopher.barnes@sernova.com
- Website: www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute “forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects", "potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should" occur are used to identify forward-looking statements. These statements reflect management’s beliefs with respect to future events and are based on information currently available to management on the date such statements were made. Many factors could cause Sernova’s actual results, performances or achievements to not be as anticipated, estimated or intended or to dier materially from those expressed or implied by the forward-looking statements contained in this news release. Such factors could include, but are not limited to, the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all; ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute its business strategy and successfully compete in the market; and the inherent risks associated with the development of biotechnology combination products generally. Many of the factors are beyond our control, including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Press Release Communiqué de presse - Mars 11, 2024 11 Mars, 2024
Sernova Announces Management Developments
LONDON, Ontario; BOSTON, Massachusetts – March 11, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage company focused on providing regenerative medicine therapeutics to
patients with chronic conditions, today announced that Nicholas J. Rossettos, CPA has joined Sernova on a
consulting basis as interim Chief Financial Officer (CFO).
With a wealth of experience in financial leadership within the biotech industry, Nick brings a fresh perspective
and a strong track record of success to the Sernova team during this transition period. The company has
initiated a formal search for a permanent CFO and is also actively recruiting additional talent to fill key
leadership roles and strengthen its senior executive team, which started with the additions of Cynthia Pussinen
as the Chief Executive Officer and Modestus Obochi as Chief Business Officer. The goal is to ensure that
Sernova has the expertise to execute its strategic vision and drive the company forward by delivering life-
changing therapies to patients worldwide.
David Swetlow, Chief Financial Officer, is no longer with the company. Mr. Swetlow’s employment was
terminated for cause after the Board received and considered findings made by independent legal counsel in
connection with an ongoing investigation into alleged misconduct. Another senior officer of the company has
been placed on administrative leave pending the final outcome of the investigation. None of the allegations, if
substantiated, are expected to materially change or impact the Company's financial statements or its reporting
obligations. The Sernova mission remains unchanged: to improve the lives of patients through groundbreaking
innovation and compassionate care. The board and the senior leadership team are deeply grateful for the
dedication and hard work of the Company’s employees, partners, and stakeholders, and remains steadfast in
its commitment to maintaining the highest standards of integrity, transparency, and accountability in all aspects
of our operations.
ABOUT SERNOVA CORP. AND THE CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead asset, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells. On implantation, The Cell Pouch forms a natural vascularized tissue environment in
the body for long-term survival and function of therapeutic cells that release essential factors that are absent or
deficient in the bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has demonstrated
its potential to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study at the University
of Chicago. In May 2022, Sernova and Evotec entered into a global strategic partnership to develop an
implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet replacement therapy. This
partnership provides Sernova a potentially unlimited supply of insulin-producing cells to treat millions of
patients with insulin-dependent diabetes (type 1 and type 2). Sernova continues to progress two additional
development programs that utilize its Cell Pouch System: a cell therapy for hypothyroid disease resulting from
thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
christopher.barnes@sernova.com
Tel: 519-902-7923
www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or "should"
occur are used to identify forward-looking statements. These statements reflect management’s beliefs with
respect to future events and are based on information currently available to management on the date such
statements were made. Many factors could cause Sernova’s actual results, performances or achievements to
not be as anticipated, estimated or intended or to differ materially from those expressed or implied by the
forward-looking statements contained in this news release. Such factors could include, but are not limited to,
the company’s ability to secure additional financing and licensing arrangements on reasonable terms, or at all;
ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies, including the timing and results of those trials; ability to obtain all necessary regulatory
approvals, or on a timely basis; ability to in-license additional complementary technologies; ability to execute
its business strategy and successfully compete in the market; and the inherent risks associated with the
development of biotechnology combination products generally. Many of the factors are beyond our control,
including those caused by, related to, or impacted by the novel coronavirus pandemic. Investors should consult
the company’s quarterly and annual filings available on www.sedarplus.ca for additional information on risks
and uncertainties relating to the forward-looking statements. Sernova expressly disclaims any intention or
obligation to update or revise any forward-looking statements, whether as a result of new information, future
events or otherwise.
Press Release Communiqué de presse - Janvier 29, 2024 29 Janvier, 2024
Sernova Provides Recap of 2023 Accomplishments and Anticipated 2024 Milestones
- Recently completed recruitment of all 7 patients in Cohort 2
- Anticipates additional data from Cohort 2 of Phase 1/2 clinical trial evaluating its enhanced 10-channel
Cell Pouch in patients with type 1 diabetes (T1D) in Q1/24
- Expects to report preclinical data supporting an IND application to evaluate the use of therapeutic cells in
combination with Cell Pouch in patients with post-operative hypothyroidism
LONDON, Ontario; Boston, Massachusetts – January 29, 2024 – Sernova Corp. (TSX:SVA) (OTCQB:SEOVF)
(FSE/XETRA:PSH), a clinical-stage company and leader in cell therapeutics, today provided a business
update, including a recap of 2023 accomplishments and a preview of certain milestones anticipated in 2024.
“In 2023, we added to the compelling and expanding set of data demonstrating the safety and efficacy of our
novel cell therapy platform for chronic diseases, including our higher capacity 10-channel Cell Pouch that is
being evaluated in our ongoing Phase 1/2 human donor islet clinical trial in T1D,” said Cynthia Pussinen, Chief
Executive Officer of Sernova. “We also made significant progress with our hemophilia A program, including
receipt of both Orphan Drug and Rare Pediatric Disease designations from the U.S. Food and Drug
Administration (FDA). Lastly, we announced an exciting preclinical research collaboration with AstraZeneca
that has the potential to significantly expand the use of the Cell Pouch in additional high-value indications.”
“Looking ahead to 2024, we anticipate additional patient data from Cohort 2 of our T1D human donor islet
trial, as well as important data from our thyroid disease program as we work to further our preclinical pipeline.
We continue to advance plans for our next T1D Phase 1/2 clinical study utilizing Evotec’s iPSC-derived islet
like clusters (ILCs) in our Cell Pouch. Use of iPSCs provides significant advantages over human donor islets,
including the ability to scale this promising treatment to commercially viable levels enabling the treatment of
millions of patients. Throughout 2024 our strategic partner, Evotec, will continue to optimize and advance the
development of iPSC derived ILCs for use in additional IND enabling studies and clinical trials. Given the
complexity around scaling-up of iPSCs and therapeutic cell manufacturing, as well as the relative nascency of
the entire advanced therapeutics field, timelines have extended, and we now anticipate initiating a clinical trial
evaluating our Cell Pouch with iPSC-derived ILCs in the fourth quarter of 2025.” Ms. Pussinen added.
“I am encouraged with our progress and believe we have built a solid foundation consisting of a portfolio of
fundamentally transformational medical treatments for patients living with chronic conditions that will result in
multiple potentially value-creating milestones this year and next,” Ms. Pussinen concluded.
2023 Achievements:
• Announced senior leadership additions, including Cynthia Pussinen as new Chief Executive Officer, and
Dr. Modestus Obochi, Ph.D., MBA, as Chief Business Officer
• Received both Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) from the
U.S. FDA for the Company’s hemophilia A program
• Announced positive interim data from Cohorts 1 and 2 of the ongoing Phase 1/2 human donor islet
clinical trial of its Cell Pouch System™ in patients with T1D at the 2023 IPITA-IXA-CTRMS Joint Congress.
Notably, five of the six patients in Cohort 1 were able to discontinue insulin therapy following islet
transplantation, and all six patients achieved HbA1c values in the non-diabetic range (<6.5%). In Cohort
2, the first six of seven planned patients received the higher capacity 10-channel Cell Pouch and five
patients received a first islet transplant. Stable fasting and stimulated serum C-peptide levels were
observed following a single islet transplant into the 10-channel Cell Pouch in the first assessable Cohort 2
patient who subsequently achieved insulin independence with a modest portal vein top-up.
• Announced positive results from its conformal coating immune protection technology program that is
used in combination with the Cell Pouch System™ and is intended to eliminate the need for chronic
immunosuppression medications.
• Presented preclinical data supporting the planned Phase 1/2 clinical trial of Evotec’s “off-the-shelf” iPSC-
derived ILCs in combination with Sernova’s Cell Pouch System for the treatment of patients with T1D.
Specifically, data demonstrated that Evotec’s iPSC-derived ILCs generated robust and durable insulin
independence with blood C-peptide levels and glucose tolerance test results equivalent to a test group
with human islets. A separate study showed sustained normalization of blood sugar levels in diabetic mice
throughout the 320-day term of the study. Human testing is anticipated to begin in late 2025.
• Announced a research collaboration with AstraZeneca to evaluate the use of Sernova’s Cell Pouch System
in combination with AstraZeneca’s novel therapeutic cells for various indications. The discovery work is
being funded and conducted by AstraZeneca.
• Completed recruitment of all 7 patients in Cohort 2 of the ongoing Phase 1/2 trial using the 10-channel
Cell Pouch.
Anticipated Select 2024 Milestones:
• Additional data from Cohort 2 of the ongoing U.S. Phase 1/2 clinical trial, which is evaluating its enhanced
10-channel Cell Pouch in patients with T1D, are expected beginning in Q1.
• Additional preclinical data to support an IND application to evaluate the Company’s autograft thyroid
cells in patients suffering from thyroid disease, with no immunosuppressive regimen required.
• Completion of thyroid disease market study validating the current market size and detailing the unmet
medical need.
• Potential engagement with additional life sciences focused companies, academic institutions and external
parties through partnership and collaboration opportunities, which could be announced over the course
of 2024.
• Additional funding to support growth through strategic alliances, credit facilities and/or institutional
equity financings with the goal of maximizing shareholder value.
Fiscal Year 2023 Financials
Today, Sernova filed its financial results on SEDAR for the fiscal year 2023.
ABOUT SERNOVA CORP. AND THE CELL POUCH SYSTEM PLATFORM FOR CELL THERAPY
Sernova Corp. is a clinical-stage biotechnology company that is developing therapeutic cell technologies for
chronic diseases, including insulin-dependent diabetes, thyroid disease, and blood disorders that include
hemophilia A. Sernova is currently focused on developing a ‘functional cure’ for insulin-dependent diabetes
with its lead asset, the Cell Pouch System, a novel implantable and scalable medical device with immune
protected therapeutic cells. On implantation, The Cell Pouch forms a natural vascularized tissue environment
in the body for long-term survival and function of therapeutic cells that release essential factors that are
absent or deficient in the bodies of patients with certain chronic diseases. Sernova’s Cell Pouch System has
demonstrated its potential to be a ‘functional cure’ for people with T1D in an ongoing Phase 1/2 clinical study
at the University of Chicago. Sernova is also advancing a proprietary technology in collaboration with the
University of Miami to shield therapeutic cells from immune system attack with the goal to eliminate the need
for chronic, systemic immunosuppression. In May 2022, Sernova and Evotec entered into a global strategic
partnership to develop an implantable off-the-shelf iPSC (induced pluripotent stem cells) based islet
replacement therapy. This partnership provides Sernova a potentially unlimited supply of insulin-producing
cells to treat millions of patients with insulin-dependent diabetes (type 1 and type 2). Sernova continues to
progress two additional development programs that utilize its Cell Pouch System: a cell therapy for
hypothyroid disease resulting from thyroid gland removal and an ex vivo lentiviral Factor VIII gene therapy for
hemophilia A.
FOR FURTHER INFORMATION, PLEASE CONTACT:
Christopher Barnes
VP, Investor Relations
Sernova Corp.
christopher.barnes@sernova.com
Tel: 519-902-7923
www.sernova.com
FORWARD-LOOKING INFORMATION
This release contains statements that, to the extent they are not recitations of historical facts, may constitute
“forward-looking statements” that involve various risks, uncertainties, and assumptions, including, without
limitation, statements regarding the prospects, plans, and objectives of the company. Wherever possible, but
not always, words such as "expects", "plans", "anticipates", "believes", "intends", "estimates", "projects",
"potential for" and similar expressions, or that events or conditions "will", "would", "may", "could" or
"should" occur are used to identify forward-looking statements. Forward looking statements in this news
release include, without limitation, statements relating to the timing of clinical trials, the timing with respect to
the receipt of data from on-going clinical trials, timing with respect to preclinical data, the completion of
thyroid disease market study, statements with respect to the potential future engagement with additional
universities, life sciences focus companies and external parties and the timing thereof.
These statements reflect the current expectations, assumptions and beliefs of management currently available
to it on the date such statements were made, including Sernova’s ability to secure additional financing and
licensing arrangements; the timing with respect to the engineering and scaling-up of Sernova’s technologies;
the ability to conduct all required preclinical and clinical studies for the company’s Cell Pouch System and or
related technologies; the timing and results of preclinical and clinical trials; the ability to obtain all necessary
regulatory approvals on a timely basis; the ability to in-license additional complementary technologies; and
the ability of Sernova to execute its business strategy, attract additional partners and successfully compete in
the market.
Although the Company believes that the assumptions inherent in these forward-looking statements are
reasonable, forward-looking statements are not a guarantee of future performance and accordingly undue
reliance should not be placed on such statements. Forward-looking statements are subject to a number of
risks and uncertainties, some that are similar to biotechnology companies and some that are unique to
Sernova. The actual results may differ materially from those expressed or implied by the forward-looking
information, and even if such actual results are realized or substantially realized, there can be no assurance
that they will have the expected consequences to, or effects on, Sernova. Sernova’s annual information form
dated January 29, 2024, its annual management's discussion and analysis for the year ended October 31,
2023, and other documents it files from time to time with securities regulatory authorities describe the risks,
uncertainties, material assumptions and other factors that could influence actual results and such factors are
incorporated herein by reference. Copies of these documents are available without charge by referring to the
company's profile on SEDAR+ at www.sedarplus.ca. All forward-looking statements speak only as of the date
on which they were made and, except as may be required by applicable securities laws, the Company
disclaims any intent or obligation to update any forward-looking statements, whether as a result of new
information, future events or results or otherwise.
Subscribe to our Newsletter Abonnez-vous à notre newsletter
By subscribing to our Newsletter you will receive Sernova’s press releases and news as they develop by email. We do not send direct mail, customized online advertising, and we do not share your personal information. We respect your privacy. En vous abonnant à notre newsletter, vous recevrez par e-mail les communiqués de presse et les actualités de Sernova au fur et à mesure de leur développement. Nous n'envoyons pas de publipostage, de publicité en ligne personnalisée et nous ne partageons pas vos informations personnelles. Nous respectons votre vie privée.
Please read our Pour plus d’informations, veuillez lire notre Privacy policypolitique de confidentialité.







